
Outdated theories of anaesthetic action
Encyclopedia
A general anaesthetic is a drug
that brings about a reversible loss of consciousness
. These drugs are generally administered by an anaesthetist/anaesthesiologist in order to induce or maintain general anaesthesia
to facilitate surgery
. General anaesthetics have been widely used in surgery since 1842 when Crawford Long for the first time administered diethyl ether to a patient and performed a painless operation. It has always been believed that general anaesthetics exert their effects (analgesia, amnesia, immobility) by modulating the activity of membrane proteins in the neuronal membrane. However, the exact location and mechanism of this action are still largely unknown although much research has been done in this area. There are a number of outdated and modern theories that attempt to explain anaesthetic action.
The concept of specific interactions between receptors and drugs first introduced by Paul Ehrlich
states that drugs act only when they are bound to their targets (receptors). However, this concept is not working well in case of general anaesthetics because:
All these common features of general anaesthetics made it hard for early researchers to believe that general anaesthetics act in a specific manner and their action on neuronal membrane was thought to be global (through nonspecific perturbation of lipid membrane of CNS neurons) rather than through specific sites.
 The nonspecific mechanism of general anaesthetic action was first proposed by Von Bibra
The nonspecific mechanism of general anaesthetic action was first proposed by Von Bibra
and Harless in 1847
.
They suggested that general anaesthetic
s may act by dissolving in the fatty fraction of brain cells and removing fatty constituents from them, thus changing activity of brain cells and inducing anaesthesia. In 1899 Hans Horst Meyer
published the first experimental evidence of the fact that anaesthetic potency is related to lipid solubility in his article entitled "Zur Theorie der Alkoholnarkose"
. Two years later a similar theory was published independently by Overton
.
Meyer compared the potency of many agents, defined as the reciprocal of the molar concentration
required to induce anaesthesia in tadpoles, with their olive oil/water partition coefficient
. He found a nearly linear relationship between potency and the partition coefficient for many types of anaesthetic molecules such as alcohol
s, aldehyde
s, ketone
s, ether
s, and ester
s.
The anaesthetic concentration required to induce anaesthesia in 50% of a population of animals (the EC50) was independent of the means by which the anaesthetic was delivered, i.e., the gas or aqueous phase
.
Meyer and Overton had discovered the striking correlation between the physical properties of general anaesthetic molecules and their potency: the greater is the lipid solubility of the compound in olive oil the greater is its anaesthetic potency
.
This correlation is true for a wide range of anaesthetics with lipid solubilities ranging over 4-5 orders of magnitude if olive oil is used as the oil phase. However, this correlation can be improved considerably in terms of both the quality of the correlation and the increased range of anaesthetics if bulk octanol
or a fully hydrated fluid lipid bilayer
is used as the “oil” phase. It was noted also that volatile anaesthetics are additive in their effects (a mixture of a half dose of two different volatile anaesthetics gave the same anaesthetic effect as a full dose of either drug alone).
Nb: The y-axis in the accompanying graph is incorrectly labelled and should read "log of Minimum Alveolar Concentration
or MAC" - The lesser the MAC the greater the potency (Halothane has a MAC of slightly less than 1 while Nitrous oxide has a MAC of around 105, halothane is much more potent and it is because the log of the MAC is plotted on the y axis that halothane has a value of 0.01 and nitrous oxide has a value of 1. The drug potency increases and the dose required to produce anaesthesia reduces as the oil:gas solubility increases.
They assumed that bulky and hydrophobic anaesthetic molecules accumulate inside the hydrophobic (or lipophilic) regions of neuronal lipid membrane causing its distortion and expansion (thickening) due to volume displacement. Accumulation of critical amounts of anaesthetic causes membrane thickening sufficient to reversibly alter function of membrane ion channels thus providing anaesthetic effect. Actual chemical structure of the anaesthetic agent per se is not important, but its molecular volume plays the major role: the more space within membrane is occupied by anaesthetic - the greater is the anaesthetic effect.
Based on this theory, in 1954 Mullins suggested that the Meyer-Overton correlation with potency can be improved if molecular volumes of anaesthetic molecules are taken into account
.
This theory existed for over 60 years and was supported by experimental fact that increases in atmospheric pressure reverse anaesthetic effect (pressure reversal effect).
Then other theories of anaesthetic action emerged mostly ‘physicochemical’ theories that took into account the diverse chemical nature of general anaesthetics and suggested that anaesthetic effect is exerted through some perturbation of the lipid bilayer. Several types of bilayer perturbations were proposed to cause anaesthetic effect (reviews
.
):
According to the lateral phase separation theory
anaesthetics exert their action by fluidizing nerve membranes to a point when phase separations in the critical lipid regions disappear. This anaesthetic-induced fluidization makes membranes less able to facilitate the conformational changes in proteins that may be the basis for such membrane events as ion gating, synaptic transmitter release, and transmitter binding to receptors.
All these outdated lipid theories generally suffer from four weaknesses
(full description see in sections below):
In conclusion it is important to point out that the correlation between lipid solubility and potency of general anaesthetics is a necessary but not sufficient condition for inferring a lipid target site. General anaesthetics could equally well be binding to hydrophobic target sites on proteins in the brain. The main reason that more polar general anaesthetics are less potent is that they have to cross the blood-brain barrier
to exert their effect on neurons in the brain.
.
Physicochemical effects of enantiomers are always identical in an achiral environment (for example in the lipid bilayer). However, in vivo enantiomers of many general anaesthetics (e.g. isoflurane
, thiopental, etomidate
) can differ greatly in their anaesthetic potency despite the similar oil/gas partition coefficients
For example, the R-(+) isomer of etomidate is 10 times more potent anaesthetic than its S-(-) isomer
.
This means that optical isomers partition identically into lipid, but have differential effects on ion channels and synaptic transmission. This objection provides a compelling evidence that the primary target for anaesthetics is not the achiral lipid bilayer itself but rather stereoselective binding sites on membrane proteins that provide a chiral
environment for specific anaesthetic-protein docking interactions
.
.
These drugs are referred to as nonimmobilizers. The existence of nonimmobilizers suggests that anaesthetics induce different components of anaesthetic effect (amnesia and immobility) by affecting different molecular targets and not just the one target (neuronal bilayer) as it was believed earlier
.
Good example of immobilizers are halogenated alkanes that are very hydrophobic, but fail to suppress movement in response to noxious stimulation at appropriate concentrations.
.
The change in body temperature of approximately 1°C is within the physiological range and clearly it is not sufficient to induce loss of consciousness per se. Thus membranes are fluidized only by large quantities of anaesthetics, but there are no changes in membrane fluidity when concentrations of anaesthetics are small and restricted to pharmacologically relevant.
of any general anaesthetic (e.g. n-alcohol
s, or alkanes) increases their lipid solubility and thereby should produce a corresponding increase in anaesthetic potency. However, addition of methylene groups cannot make anaesthetic increasingly potent without limit and at a certain chain length (cutoff chain length) the addition of just one methylene group causes the molecule to lose its ability to anaesthetise. For the n-alcohols the cutoff occurs at a carbon chain length of about 13
and for the n-alkanes at a chain length of between 6 and 10, depending on the species
.
If general anaesthetics disrupt ion channels by partitioning into and perturbing the lipid bilayer, then one would expect that their solubility in lipid bilayers would also display the cutoff effect. However, partitioning of alcohols into lipid bilayers does not display a cutoff for long-chain alcohols from n-decanol to n-pentadecanol. A plot of chain length vs. the logarithm of the lipid bilayer/buffer partition coefficient K is linear, with the addition of each methylene group causing a change in the Gibbs free energy
of -3.63 kJ/mol.
The cutoff effect was first interpreted as evidence that anaesthetics exert their effect not by acting globally on membrane lipids but rather by binding directly to hydrophobic pockets of well-defined volumes in proteins. As the alkyl chain grows, the anaesthetic fills more of the hydrophobic pocket and binds with greater affinity. When the molecule is too large to be entirely accommodated by the hydrophobic pocket, the binding affinity no longer increases with increasing chain length. Thus the volume of the n-alkanol chain at the cutoff length provides an estimate of the binding site volume. This objection provided the basis for protein hypothesis of anaesthetic effect (see below).
However, cutoff effect can still be explained in the frame of lipid hypothesis
.
In short-chain alkanols (A) segments of the chain are rather rigid (in terms of conformational enthropy) and very close to hydroxyl group tethered to aqueous interfacial region ("buoy"). Consequently, these segments efficiently redistribute lateral stresses from the bilayer interior toward the interface. In long-chain alkanols (B) hydrocarbon chain segments are located further from hydroxyl group and are more flexible than in short-chain alkanols. Efficiency of pressure redistribution decreases as the length of hydrocarbon chain increases until anaesthetic potency is lost at some point. It was proposed that polyalkanols (C) will have anaesthetic effect similar to short-chain 1-alkanols if the chain length between two neighbouring hydroxyl groups is smaller than the cutoff
.
This idea was supported by the experimental evidence because polyhydroxyalkanes 1,6,11,16-hexadecanetetraol and 2,7,12,17-octadecanetetraol exhibited significant anaesthetic potency as was originally proposed
.
.
Each bilayer membrane has distinct profile of how lateral pressures are distributed within it. Most membrane proteins especially ion channels are sensitive to changes in this lateral pressure distribution profile. These lateral stresses are rather large and vary with depth within the membrane. According to modern lipid hypothesis change in membrane lateral pressure profile shifts the conformational equilibrium of certain membrane proteins known to be affected by clinical concentrations of anaesthetics such as ligand-gated ion channels. This mechanism is also nonspecific because potency of the anaesthetic is determined not by its actual chemical structure, but by positional and orientational distribution of its segments and bonds of within the bilayer. However, it was not obvious what is the exact molecular mechanism...
Detailed mechanism of general anaesthesia was suggested and investigated using lattice statistical thermodynamics. It was proposed that incorporation of amphiphilic and other interfacially active solutes (e.g. general anaesthetics) into the bilayer increases the lateral pressure selectively near the aqueous interfaces, which is compensated by decrease in lateral pressure toward the centre of the bilayer. Calculations showed that general anaesthesia likely involves inhibition of the opening of the ion channel in a postsynaptic ligand-gated membrane protein by the following mechanism:
Thus according to the modern lipid hypothesis anaesthetics do not act directly on their membrane protein targets, but rather perturb specialized lipid matrices at the protein-lipid interface, which act as mediators. This is a new kind of transduction mechanism, different from the usual key-lock interaction of ligand and receptor, where anaesthetic (ligand) affects the function of membrane proteins by binding to the specific site on the protein. Thus some membrane proteins are proposed to be sensitive to their lipid environment.
Slightly different detailed molecular mechanism of how bilayer perturbation can influence the ion-channel was proposed in the same year. Oleamide (fatty acid amide of oleic acid) is an endogenous anaesthetic found in vivo (in the cat’s brain) and it is known to potentiate sleep and lower temperature of the body through closing gap junction channel connexon
.
The detailed mechanism is shown on the picture: the well ordered lipid(green)/cholesterol(yellow) ring that exists around connexon (magenta) becomes disordered on treatment with anaesthetic (red triangles), promoting a closure of connexon ion channel. This decreases brain activity and induces lethargy and anaesthetic effect.
demonstrated that the Meyer-Overton correlation can be reproduced using a soluble protein. They found that two classes of proteins are inactivated by clinical doses of anaesthetic in the total absence of lipid. These are luciferase
s, which are used by bioluminescent
animals and bacteria to produce light,
and cytochrome P450
, which is a group of heme
proteins that hydroxylate a diverse group of compounds, including fatty acid
s, steroid
s, and xenobiotic
s such as phenobarbital
. Remarkably, inhibition of these proteins by general anaesthetics was directly correlated with their anaesthetic potencies. Luciferase inhibition also exhibits a long-chain alcohol cutoff, which is related to the size of the anaesthetic-binding pocket
.
These observations were important because they demonstrated that general anaesthetics may also interact with hydrophobic protein sites of certain proteins, rather than affect membrane proteins indirectly through nonspecific interactions with lipid bilayer as mediator
.
It was shown that anaesthetics alter the functions of many cytoplasmic signalling proteins, including protein kinase C,
however, the proteins considered the most likely molecular targets of anaesthetics are ion channels.
According to this theory general anaesthetics are much more selective than in the frame of lipid hypothesis and they bind directly only to small number of targets in CNS mostly ligand(neurotransmitter)-gated ion channels in synapse and G-protein coupled receptors altering their ion flux. Particularly Cys-loop receptors
are plausible targets for general anaesthetics that bind at the interface between the subunits. The Cys-loop receptor superfamily includes inhibitory receptors (GABA A, GABA C, glycine receptors) and excitatory receptors (acetylcholine receptor and 5-HT3 serotonin receptor). General anaesthetics can inhibit the channel functions of excitatory receptors or potentiate functions of inhibitory receptors, respectively. Although protein targets for anaesthetics have been partly identified the exact nature of general anaesthetic-protein interactions still remains a mystery.
It was initially hypothesized that general anaesthetic binds to its target ion channel by a key-lock mechanism and changes its structure dramatically from open to closed conformation or vice versa. However, there is lots of evidence accumulated against direct key-lock interaction of membrane proteins with general anaesthetics
.
Various studies have shown that low affinity drugs including inhaled general anaesthetics do not usually interact with their target proteins via specific lock-and-key binding mechanism because they do not change molecular structures of transmembrane receptors, ion channels and globular proteins.
Based on these experimental facts and some computer simulations modern version of protein hypothesis was proposed
.
Proteins of four-α-helix bundle structural motif served as models of monomer of pentameric Cys-loop receptor because binding pockets of inhaled anaesthetics are believed to be within transmembrane four-α-helix bundles of Cys-loop receptors
.
Inhaled general anaesthetic does not change structure of membrane channel but changes its dynamics especially dynamics in the flexible loops that connect α-helices in a bundle and are exposed to the membrane-water interface. It is a well known fact that dynamics of protein in microsecond-millisecond timescale is often coupled with functions of the protein. Thus it was logical to propose that since inhaled general anaesthetics do not change protein structure they may exert their effect on proteins by modulating protein dynamics in a slow microsecond-millisecond timescale and/or by disrupting the modes of motion essential for function of this protein. Normal interactions between residues in protein regions (loops) at the water-lipid interface that play critical roles in protein functions and agonist binding may be disrupted by general anaesthetic. Interactions within the same loop or between different loops may be disrupted by anaesthetics and ultimately functions of Cys-loop receptors may be altered.
Drug
A drug, broadly speaking, is any substance that, when absorbed into the body of a living organism, alters normal bodily function. There is no single, precise definition, as there are different meanings in drug control law, government regulations, medicine, and colloquial usage.In pharmacology, a...
that brings about a reversible loss of consciousness
Consciousness
Consciousness is a term that refers to the relationship between the mind and the world with which it interacts. It has been defined as: subjectivity, awareness, the ability to experience or to feel, wakefulness, having a sense of selfhood, and the executive control system of the mind...
. These drugs are generally administered by an anaesthetist/anaesthesiologist in order to induce or maintain general anaesthesia
General anaesthesia
General anaesthesia is a state of unconsciousness and loss of protective reflexes resulting from the administration of one or more general anaesthetic agents...
to facilitate surgery
Surgery
Surgery is an ancient medical specialty that uses operative manual and instrumental techniques on a patient to investigate and/or treat a pathological condition such as disease or injury, or to help improve bodily function or appearance.An act of performing surgery may be called a surgical...
. General anaesthetics have been widely used in surgery since 1842 when Crawford Long for the first time administered diethyl ether to a patient and performed a painless operation. It has always been believed that general anaesthetics exert their effects (analgesia, amnesia, immobility) by modulating the activity of membrane proteins in the neuronal membrane. However, the exact location and mechanism of this action are still largely unknown although much research has been done in this area. There are a number of outdated and modern theories that attempt to explain anaesthetic action.
The concept of specific interactions between receptors and drugs first introduced by Paul Ehrlich
states that drugs act only when they are bound to their targets (receptors). However, this concept is not working well in case of general anaesthetics because:
- Molecular structures of general anaesthetics widely used in medicine are very simple and diverse so that there is no obvious structure–activity relationship (see structures of general anaesthetics widely used in medicine: 1-ethanol, 2- chloroform, 3-diethylether, 4-fluroxene, 5-halothane, 6-metheoxyflurane, 7- enflurane, 8-isoflurane, 9-desflurane, 10-sevoflurane)
- Most general anaesthetics have remarkably weak affinity for their targets acting at much higher concentrations than most other drugs so that diverse side effects are inevitable [ref].
All these common features of general anaesthetics made it hard for early researchers to believe that general anaesthetics act in a specific manner and their action on neuronal membrane was thought to be global (through nonspecific perturbation of lipid membrane of CNS neurons) rather than through specific sites.
Lipid solubility-anaesthetic potency correlation (the Meyer-Overton correlation)

Ernst von Bibra
Dr. Ernst Freiherr von Bibra was a German Naturalist and author...
and Harless in 1847
.
They suggested that general anaesthetic
General anaesthetic
A general anaesthetic is a drug that brings about a reversible loss of consciousness. These drugs are generally administered by an anaesthesia provider to induce or maintain general anaesthesia to facilitate surgery...
s may act by dissolving in the fatty fraction of brain cells and removing fatty constituents from them, thus changing activity of brain cells and inducing anaesthesia. In 1899 Hans Horst Meyer
Hans Horst Meyer
Hans Horst Meyer was a German pharmacologist. He studied medicine and did research in pharmacology. The Meyer-Overton hypothesis on the mode of action on general anaesthetics is partially named after him...
published the first experimental evidence of the fact that anaesthetic potency is related to lipid solubility in his article entitled "Zur Theorie der Alkoholnarkose"
. Two years later a similar theory was published independently by Overton
.
Meyer compared the potency of many agents, defined as the reciprocal of the molar concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
required to induce anaesthesia in tadpoles, with their olive oil/water partition coefficient
Partition coefficient
In chemistry and the pharmaceutical sciences, a partition- or distribution coefficient is the ratio of concentrations of a compound in the two phases of a mixture of two immiscible solvents at equilibrium. The terms "gas/liquid partition coefficient" and "air/water partition coefficient" are...
. He found a nearly linear relationship between potency and the partition coefficient for many types of anaesthetic molecules such as alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s, aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s, ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s, ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
s, and ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s.
The anaesthetic concentration required to induce anaesthesia in 50% of a population of animals (the EC50) was independent of the means by which the anaesthetic was delivered, i.e., the gas or aqueous phase
.
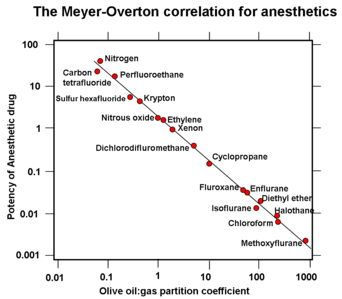
Meyer and Overton had discovered the striking correlation between the physical properties of general anaesthetic molecules and their potency: the greater is the lipid solubility of the compound in olive oil the greater is its anaesthetic potency
.
This correlation is true for a wide range of anaesthetics with lipid solubilities ranging over 4-5 orders of magnitude if olive oil is used as the oil phase. However, this correlation can be improved considerably in terms of both the quality of the correlation and the increased range of anaesthetics if bulk octanol
or a fully hydrated fluid lipid bilayer
is used as the “oil” phase. It was noted also that volatile anaesthetics are additive in their effects (a mixture of a half dose of two different volatile anaesthetics gave the same anaesthetic effect as a full dose of either drug alone).
Nb: The y-axis in the accompanying graph is incorrectly labelled and should read "log of Minimum Alveolar Concentration
Minimum alveolar concentration
Minimum alveolar concentration or MAC is a concept used to compare the strengths, or potency, of anaesthetic vapours; in simple terms, it is defined as the concentration of the vapour in the lungs that is needed to prevent movement in 50% of subjects in response to surgical stimulus...
or MAC" - The lesser the MAC the greater the potency (Halothane has a MAC of slightly less than 1 while Nitrous oxide has a MAC of around 105, halothane is much more potent and it is because the log of the MAC is plotted on the y axis that halothane has a value of 0.01 and nitrous oxide has a value of 1. The drug potency increases and the dose required to produce anaesthesia reduces as the oil:gas solubility increases.
Outdated lipid hypotheses of general anaesthetic action
From the correlation between lipid solubility and anaesthetic potency, both Meyer and Overton had surmised a unitary mechanism of general anaesthesia. They assumed that solubilization of lipophilic general anaesthetic in lipid bilayer of the neuron causes its malfunction and anaesthetic effect when critical concentration of anaesthetic is reached. Later in 1973 Miller and Smith suggested the critical volume hypothesis also called lipid bilayer expansion hypothesis.They assumed that bulky and hydrophobic anaesthetic molecules accumulate inside the hydrophobic (or lipophilic) regions of neuronal lipid membrane causing its distortion and expansion (thickening) due to volume displacement. Accumulation of critical amounts of anaesthetic causes membrane thickening sufficient to reversibly alter function of membrane ion channels thus providing anaesthetic effect. Actual chemical structure of the anaesthetic agent per se is not important, but its molecular volume plays the major role: the more space within membrane is occupied by anaesthetic - the greater is the anaesthetic effect.
Based on this theory, in 1954 Mullins suggested that the Meyer-Overton correlation with potency can be improved if molecular volumes of anaesthetic molecules are taken into account
.
This theory existed for over 60 years and was supported by experimental fact that increases in atmospheric pressure reverse anaesthetic effect (pressure reversal effect).
Then other theories of anaesthetic action emerged mostly ‘physicochemical’ theories that took into account the diverse chemical nature of general anaesthetics and suggested that anaesthetic effect is exerted through some perturbation of the lipid bilayer. Several types of bilayer perturbations were proposed to cause anaesthetic effect (reviews
.
):
- changes in phase separation
- changes in bilayer thickness
- changes in order parameters
- changes in curvature elasticity
According to the lateral phase separation theory
anaesthetics exert their action by fluidizing nerve membranes to a point when phase separations in the critical lipid regions disappear. This anaesthetic-induced fluidization makes membranes less able to facilitate the conformational changes in proteins that may be the basis for such membrane events as ion gating, synaptic transmitter release, and transmitter binding to receptors.
All these outdated lipid theories generally suffer from four weaknesses
(full description see in sections below):
- Stereoisomers of an anaesthetic drug have very different anaesthetic potency whereas their oil/gas partition coefficients are similar
- Certain highly lipid soluble drugs expected to act as anaesthetics, exert convulsive effect instead of anaesthetic effect and therefore such drugs were termed nonimmobilizers.
- General anaesthetic-induced changes in membrane density and fluidity are so small that relatively small increases in temperature can mimic these effects on membrane fluidity and density without causing anaesthesia.
- Cutoff effect - addition of methylene groups to a homologous series of long chain alcohols, or alkanes, increases their lipid solubility and should thereby produce a corresponding increase in anaesthetic potency, but it was observed only to a certain "cutoff" length of the chain.
In conclusion it is important to point out that the correlation between lipid solubility and potency of general anaesthetics is a necessary but not sufficient condition for inferring a lipid target site. General anaesthetics could equally well be binding to hydrophobic target sites on proteins in the brain. The main reason that more polar general anaesthetics are less potent is that they have to cross the blood-brain barrier
Blood-brain barrier
The blood–brain barrier is a separation of circulating blood and the brain extracellular fluid in the central nervous system . It occurs along all capillaries and consists of tight junctions around the capillaries that do not exist in normal circulation. Endothelial cells restrict the diffusion...
to exert their effect on neurons in the brain.
1. Stereoisomers of an anaesthetic drug
Stereoisomers that represent mirror images of each other are termed enantiomers or optical isomers (for example, the isomers of R-(+)- and S-(-)-etomidate).
Physicochemical effects of enantiomers are always identical in an achiral environment (for example in the lipid bilayer). However, in vivo enantiomers of many general anaesthetics (e.g. isoflurane
Isoflurane
Isoflurane is a halogenated ether used for inhalational anesthesia. Together with enflurane and halothane, it replaced the flammable ethers used in the pioneer days of surgery. Its name comes from being a structural isomer of enflurane, hence they have the same empirical formula...
, thiopental, etomidate
Etomidate
Etomidate is a short acting intravenous anaesthetic agent used for the induction of general anaesthesia and for sedation for short procedures such as reduction of dislocated joints, tracheal intubation and cardioversion...
) can differ greatly in their anaesthetic potency despite the similar oil/gas partition coefficients
For example, the R-(+) isomer of etomidate is 10 times more potent anaesthetic than its S-(-) isomer
.
This means that optical isomers partition identically into lipid, but have differential effects on ion channels and synaptic transmission. This objection provides a compelling evidence that the primary target for anaesthetics is not the achiral lipid bilayer itself but rather stereoselective binding sites on membrane proteins that provide a chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
environment for specific anaesthetic-protein docking interactions
.
2. Nonimmobilizers
All general anaesthetics induce immobilization (absence of movement in response to noxious stimuli) through depression of spinal cord functions, whereas their amnesic actions are exerted within the brain. According to the Meyer-Overton correlation the anaesthetic potency of the drug is directly proportional to its lipid solubility, however, there are many compounds that do not satisfy this rule. These drugs are strikingly similar to potent general anaesthetics and are predicted to be potent anaesthetics based on their lipid solubility, but they exert only one constituent of the anaesthetic action (amnesia) and do not suppress movement (i.e. do not depress spinal cord functions) as all anaesthetics do.
These drugs are referred to as nonimmobilizers. The existence of nonimmobilizers suggests that anaesthetics induce different components of anaesthetic effect (amnesia and immobility) by affecting different molecular targets and not just the one target (neuronal bilayer) as it was believed earlier
.
Good example of immobilizers are halogenated alkanes that are very hydrophobic, but fail to suppress movement in response to noxious stimulation at appropriate concentrations.
3. General anaesthetic-induced changes in membrane density and fluidity
Experimental studies have shown that general anaesthetics including ethanol are potent fluidizers of natural and artificial membranes. However, changes in membrane density and fluidity in the presence of clinical concentrations of general anaesthetics are so small that relatively small increases in temperature (~1°C) can mimic them without causing anaesthesia.
The change in body temperature of approximately 1°C is within the physiological range and clearly it is not sufficient to induce loss of consciousness per se. Thus membranes are fluidized only by large quantities of anaesthetics, but there are no changes in membrane fluidity when concentrations of anaesthetics are small and restricted to pharmacologically relevant.
4. Cutoff effect
According to the Meyer-Overton correlation, the addition of methylene groups to a homologous seriesHomologous series
In chemistry, a homologous series is a series of compounds with a similar general formula, possessing similar chemical properties due to the presence of the same functional group, and showing a gradation in physical properties as a result of increase in molecular size and mass...
of any general anaesthetic (e.g. n-alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s, or alkanes) increases their lipid solubility and thereby should produce a corresponding increase in anaesthetic potency. However, addition of methylene groups cannot make anaesthetic increasingly potent without limit and at a certain chain length (cutoff chain length) the addition of just one methylene group causes the molecule to lose its ability to anaesthetise. For the n-alcohols the cutoff occurs at a carbon chain length of about 13
and for the n-alkanes at a chain length of between 6 and 10, depending on the species
.
If general anaesthetics disrupt ion channels by partitioning into and perturbing the lipid bilayer, then one would expect that their solubility in lipid bilayers would also display the cutoff effect. However, partitioning of alcohols into lipid bilayers does not display a cutoff for long-chain alcohols from n-decanol to n-pentadecanol. A plot of chain length vs. the logarithm of the lipid bilayer/buffer partition coefficient K is linear, with the addition of each methylene group causing a change in the Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
of -3.63 kJ/mol.
The cutoff effect was first interpreted as evidence that anaesthetics exert their effect not by acting globally on membrane lipids but rather by binding directly to hydrophobic pockets of well-defined volumes in proteins. As the alkyl chain grows, the anaesthetic fills more of the hydrophobic pocket and binds with greater affinity. When the molecule is too large to be entirely accommodated by the hydrophobic pocket, the binding affinity no longer increases with increasing chain length. Thus the volume of the n-alkanol chain at the cutoff length provides an estimate of the binding site volume. This objection provided the basis for protein hypothesis of anaesthetic effect (see below).
However, cutoff effect can still be explained in the frame of lipid hypothesis
.
In short-chain alkanols (A) segments of the chain are rather rigid (in terms of conformational enthropy) and very close to hydroxyl group tethered to aqueous interfacial region ("buoy"). Consequently, these segments efficiently redistribute lateral stresses from the bilayer interior toward the interface. In long-chain alkanols (B) hydrocarbon chain segments are located further from hydroxyl group and are more flexible than in short-chain alkanols. Efficiency of pressure redistribution decreases as the length of hydrocarbon chain increases until anaesthetic potency is lost at some point. It was proposed that polyalkanols (C) will have anaesthetic effect similar to short-chain 1-alkanols if the chain length between two neighbouring hydroxyl groups is smaller than the cutoff
.
This idea was supported by the experimental evidence because polyhydroxyalkanes 1,6,11,16-hexadecanetetraol and 2,7,12,17-octadecanetetraol exhibited significant anaesthetic potency as was originally proposed
.
Modern lipid hypothesis
Modern version of lipid hypothesis states that anaesthetic effect happens if solubilization of general anaesthetic in the bilayer causes redistribution of membrane lateral pressures.
Each bilayer membrane has distinct profile of how lateral pressures are distributed within it. Most membrane proteins especially ion channels are sensitive to changes in this lateral pressure distribution profile. These lateral stresses are rather large and vary with depth within the membrane. According to modern lipid hypothesis change in membrane lateral pressure profile shifts the conformational equilibrium of certain membrane proteins known to be affected by clinical concentrations of anaesthetics such as ligand-gated ion channels. This mechanism is also nonspecific because potency of the anaesthetic is determined not by its actual chemical structure, but by positional and orientational distribution of its segments and bonds of within the bilayer. However, it was not obvious what is the exact molecular mechanism...
Detailed mechanism of general anaesthesia was suggested and investigated using lattice statistical thermodynamics. It was proposed that incorporation of amphiphilic and other interfacially active solutes (e.g. general anaesthetics) into the bilayer increases the lateral pressure selectively near the aqueous interfaces, which is compensated by decrease in lateral pressure toward the centre of the bilayer. Calculations showed that general anaesthesia likely involves inhibition of the opening of the ion channel in a postsynaptic ligand-gated membrane protein by the following mechanism:
- Channel is trying to open in the response to nerve impulse thus increasing the cross-sectional area of the protein more near the aqueous interface than in the middle of the bilayer,
- Then the anaesthetic-induced increase in lateral pressure near the interface shifts the protein conformational equilibrium to back to the closed state, since channel opening will require greater work against the higher pressure at interface. This is the first hypothesis that provided not just correlations of potency with structural or thermodynamic properties, but a detailed mechanistic and thermodynamic understanding of anaesthesia.
Thus according to the modern lipid hypothesis anaesthetics do not act directly on their membrane protein targets, but rather perturb specialized lipid matrices at the protein-lipid interface, which act as mediators. This is a new kind of transduction mechanism, different from the usual key-lock interaction of ligand and receptor, where anaesthetic (ligand) affects the function of membrane proteins by binding to the specific site on the protein. Thus some membrane proteins are proposed to be sensitive to their lipid environment.
Slightly different detailed molecular mechanism of how bilayer perturbation can influence the ion-channel was proposed in the same year. Oleamide (fatty acid amide of oleic acid) is an endogenous anaesthetic found in vivo (in the cat’s brain) and it is known to potentiate sleep and lower temperature of the body through closing gap junction channel connexon
.
The detailed mechanism is shown on the picture: the well ordered lipid(green)/cholesterol(yellow) ring that exists around connexon (magenta) becomes disordered on treatment with anaesthetic (red triangles), promoting a closure of connexon ion channel. This decreases brain activity and induces lethargy and anaesthetic effect.
Membrane protein hypothesis of general anaesthetic action
In the early 1980s, Franks and Liebdemonstrated that the Meyer-Overton correlation can be reproduced using a soluble protein. They found that two classes of proteins are inactivated by clinical doses of anaesthetic in the total absence of lipid. These are luciferase
Luciferase
Luciferase is a generic term for the class of oxidative enzymes used in bioluminescence and is distinct from a photoprotein. One famous example is the firefly luciferase from the firefly Photinus pyralis. "Firefly luciferase" as a laboratory reagent usually refers to P...
s, which are used by bioluminescent
Bioluminescence
Bioluminescence is the production and emission of light by a living organism. Its name is a hybrid word, originating from the Greek bios for "living" and the Latin lumen "light". Bioluminescence is a naturally occurring form of chemiluminescence where energy is released by a chemical reaction in...
animals and bacteria to produce light,
and cytochrome P450
, which is a group of heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
proteins that hydroxylate a diverse group of compounds, including fatty acid
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
s, steroid
Steroid
A steroid is a type of organic compound that contains a characteristic arrangement of four cycloalkane rings that are joined to each other. Examples of steroids include the dietary fat cholesterol, the sex hormones estradiol and testosterone, and the anti-inflammatory drug dexamethasone.The core...
s, and xenobiotic
Xenobiotic
A xenobiotic is a chemical which is found in an organism but which is not normally produced or expected to be present in it. It can also cover substances which are present in much higher concentrations than are usual...
s such as phenobarbital
Phenobarbital
Phenobarbital or phenobarbitone is a barbiturate, first marketed as Luminal by Friedr. Bayer et comp. It is the most widely used anticonvulsant worldwide, and the oldest still commonly used. It also has sedative and hypnotic properties but, as with other barbiturates, has been superseded by the...
. Remarkably, inhibition of these proteins by general anaesthetics was directly correlated with their anaesthetic potencies. Luciferase inhibition also exhibits a long-chain alcohol cutoff, which is related to the size of the anaesthetic-binding pocket
.
These observations were important because they demonstrated that general anaesthetics may also interact with hydrophobic protein sites of certain proteins, rather than affect membrane proteins indirectly through nonspecific interactions with lipid bilayer as mediator
.
It was shown that anaesthetics alter the functions of many cytoplasmic signalling proteins, including protein kinase C,
however, the proteins considered the most likely molecular targets of anaesthetics are ion channels.
According to this theory general anaesthetics are much more selective than in the frame of lipid hypothesis and they bind directly only to small number of targets in CNS mostly ligand(neurotransmitter)-gated ion channels in synapse and G-protein coupled receptors altering their ion flux. Particularly Cys-loop receptors
are plausible targets for general anaesthetics that bind at the interface between the subunits. The Cys-loop receptor superfamily includes inhibitory receptors (GABA A, GABA C, glycine receptors) and excitatory receptors (acetylcholine receptor and 5-HT3 serotonin receptor). General anaesthetics can inhibit the channel functions of excitatory receptors or potentiate functions of inhibitory receptors, respectively. Although protein targets for anaesthetics have been partly identified the exact nature of general anaesthetic-protein interactions still remains a mystery.
It was initially hypothesized that general anaesthetic binds to its target ion channel by a key-lock mechanism and changes its structure dramatically from open to closed conformation or vice versa. However, there is lots of evidence accumulated against direct key-lock interaction of membrane proteins with general anaesthetics
.
Various studies have shown that low affinity drugs including inhaled general anaesthetics do not usually interact with their target proteins via specific lock-and-key binding mechanism because they do not change molecular structures of transmembrane receptors, ion channels and globular proteins.
Based on these experimental facts and some computer simulations modern version of protein hypothesis was proposed
.
Proteins of four-α-helix bundle structural motif served as models of monomer of pentameric Cys-loop receptor because binding pockets of inhaled anaesthetics are believed to be within transmembrane four-α-helix bundles of Cys-loop receptors
.
Inhaled general anaesthetic does not change structure of membrane channel but changes its dynamics especially dynamics in the flexible loops that connect α-helices in a bundle and are exposed to the membrane-water interface. It is a well known fact that dynamics of protein in microsecond-millisecond timescale is often coupled with functions of the protein. Thus it was logical to propose that since inhaled general anaesthetics do not change protein structure they may exert their effect on proteins by modulating protein dynamics in a slow microsecond-millisecond timescale and/or by disrupting the modes of motion essential for function of this protein. Normal interactions between residues in protein regions (loops) at the water-lipid interface that play critical roles in protein functions and agonist binding may be disrupted by general anaesthetic. Interactions within the same loop or between different loops may be disrupted by anaesthetics and ultimately functions of Cys-loop receptors may be altered.

