
Organoiridium compound
Encyclopedia
3.png)
Iridium
Iridium is the chemical element with atomic number 77, and is represented by the symbol Ir. A very hard, brittle, silvery-white transition metal of the platinum family, iridium is the second-densest element and is the most corrosion-resistant metal, even at temperatures as high as 2000 °C...
-carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
s. Compounds with Ir-C bonds are found in oxidation states from 0 to V. For example, oxidation state zero is found in tetrairidium dodecacarbonyl
Tetrairidium dodecacarbonyl
Tetrairidium dodecacarbonyl is the chemical compound with the formula Ir412. This tetrahedral cluster is the most common and most stable "binary" carbonyl of iridium. This air-stable species is only poorly soluble in organic solvents. It has been used to prepare bimetallic clusters and catalysts,...
, , which is the most common and stable binary carbonyl
Metal carbonyl
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. These complexes may be homoleptic, that is containing only CO ligands, such as nickel carbonyl , but more commonly metal carbonyls contain a mix of ligands, such as Re3Cl...
of iridium. In this compound, each of the iridium atoms is bonded to the other three, forming a tetrahedral cluster. Some organometallic Ir(I) compounds are notable enough to be named after their discoverers. One is Vaska's complex
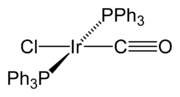
Vaska's complex
Vaska's complex is the trivial name for the chemical compound trans-chlorocarbonylbisiridium, which has the formula IrCl[P3]2. This square planar diamagnetic organometallic complex consists of a central iridium atom bound to two mutually trans triphenylphosphine ligands, carbon monoxide, and a...
, , which has the unusual property of reversibly binding to dioxygen molecule, O2. Another one is Crabtree's catalyst
Crabtree's catalyst
Crabtree's catalyst is the name given to a complex of iridium with 1,5-cyclooctadiene, tris-cyclohexylphosphine, and pyridine. It is a homogeneous catalyst for hydrogenation reactions, developed by Robert H. Crabtree, a professor at Yale University...
, a homogeneous catalyst for hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
reactions. Iridium(I) complexes are both square planar
Square planar
The square planar molecular geometry in chemistry describes the stereochemistry that is adopted by certain chemical compounds...
, d8 complexes, with a total of 16 valence electron
Valence electron
In chemistry, valence electrons are the electrons of an atom that can participate in the formation of chemical bonds with other atoms. Valence electrons are the "own" electrons, present in the free neutral atom, that combine with valence electrons of other atoms to form chemical bonds. In a single...
s, which accounts for their reactivity. Many organoiridium compounds are generated from pentamethylcyclopentadienyl iridium dichloride dimer
Pentamethylcyclopentadienyl iridium dichloride dimer
Pentamethylcyclopentadienyl iridium dichloride is an organometallic compound with the formula []2, commonly abbreviated [Cp*IrCl2]2 This bright orange air-stable diamagnetic solid is a reagent in organometallic chemistry....
.
Uses
The dominant application of organoiridium complexes is as catalysts in the Cativa processCativa process
The Cativa process is a method for the production of acetic acid by the carbonylation of methanol. The technology, which is similar to the Monsanto process, was developed by BP Chemicals and is under license by BP Plc...
for carbonylation
Carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry.-Organic chemistry:...
of methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
to produce acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
Iridium is competitive with rhodium for this large-scale application because of lower operating costs.
In the academic laboratories, iridium complexes are widely studied because its complexes promote C-H activation, but such reactions are not employed in any commercial process. Iridium complexes are highly active for hydrogenation both directly and via transfer hydrogenation
Transfer hydrogenation
Transfer hydrogenation is the addition of hydrogen to a molecule from a source other than gaseous H2. It is applied in industry and in organic synthesis, in part because of the inconvenience and expense of using gaseous H2...
. The asymmetric versions of these reactions are widely studied.
Iridium complex
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
es such as Ir(mppy)3 have been examined in the context of phosphorescent organic light-emitting diode technology to increase the internal quantum efficiency from 25% to almost 100% by harvesting emission from triplet state
Triplet state
A spin triplet is a set of three quantum states of a system, each with total spin S = 1 . The system could consist of a single elementary massive spin 1 particle such as a W or Z boson, or be some multiparticle state with total spin angular momentum of one.In physics, spin is the angular momentum...
s.
See also
- :Category:Iridium compounds
- Other chemistries of carbon with other elements in the periodic table.

