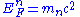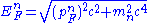Nuclear drip line
Encyclopedia
In nuclear physics
, the boundaries for nuclear particle-stability are conceptualized as drip lines. The nuclear landscape is understood by plotting boxes, each of which represents a unique nuclear species, on a graph with the number of neutrons increasing on the abscissa
and number of protons increasing along the ordinate
, which is commonly referred to as the table of nuclides, being to nuclear physics
what the more commonly known periodic table of the elements is to chemistry
. However, an arbitrary combination of protons and neutrons does not necessarily yield a stable nucleus
, and ultimately when continuing to add more of the same type of nucleon
s to a given nucleus, the newly formed nucleus will essentially undergo immediate decay where a nucleon of the same isospin quantum number
(proton or neutron) is emitted; colloquially the nucleon has 'leaked' or 'dripped' out of the target nucleus, hence giving rise to the term "drip line". The nucleons drip out of such unstable nuclei for the same reason that water drips from a leaking faucet: the droplet, or nucleon in this case, sees a lower potential which is great enough to overcome surface tension in the case of water droplets, and the strong nuclear force in the case of proton emission
or alpha decay
. As nucleons are quantized
, then only integer values
are plotted on the table of isotopes, indicating that the drip line is not linear
but instead looks like a step function
up close.
There are drip lines for protons, neutrons, and alpha particles, and these all play important roles in nuclear astrophysics
.
in particle physics
).
While the concept of nuclear drip lines is very simple in principle because naturally occurring isotopes on Earth do not undergo proton or neutron emission, and to the complexity of the alpha drip line, the terms are not introduced in some undergraduate textbooks on nuclear physics. The idea may become more common place with the advent of radioactive ion beam
accelerators in the late 1980s, which are allowing nuclear physicists to really probe the limits of nuclear stability. To understand the concept, one only needs to apply the principle of conservation of energy to nuclear binding energy
.
is a type of particle decay; alpha-decay can be considered either a form of particle decay or, less frequently, a special case of nuclear fission
. The timescale for alpha decay can be much longer than for proton or neutron emission owing to the high Coulomb barrier seen by an alpha-cluster in a nucleus. Although there are no naturally occurring nuclei on Earth which undergo proton emission or neutron emission
, such nuclei can be created, for example, in the laboratory with accelerators
or naturally in stars
. Such particle decays are not commonly known because particle decay is governed by the nuclear strong force
, as well as the Coulomb force in the case of charged particles, which can act very quickly (femtoseconds or less). In nuclear physics terms, nuclei that are outside the drip lines are particle-unbound and considered not to exist, because they can only exist in the energy continuum rather than in the discrete quantized states we are familiar with. In a discussion of the proton and neutron drip lines, one nomenclatural convenience is to regard beta-unstable nuclei as stable (strictly speaking they are particle-stable), due to the significant difference in the time-scales of these two different decay modes. Thus, the only type of nuclei which are longer lived and undergo proton or neutron emission are in the class of beta-delayed decays, where first the isospin of one nucleon is reversed (proton to neutron or vice versa) via beta-decay, and then if the particle separation energy is non-positive, the daughter nucleus will undergo particle decay. Most naturally occurring γ-sources are technically β-delayed γ-decay, so this concept should be familiar; some gamma-sources are α-delayed but these are generally categorized with other alpha-sources.
. In addition to this, however, there is an energy due to degeneracy: for instance a nucleon with energy E1 will be forced to a higher energy E2 if all the lower energy states are filled. This is because nucleons are fermions and obey Fermi-Dirac statistics
. The work done in putting this nucleon to a higher energy level results in a pressure which is the degeneracy pressure. So we can view the energy of a nucleon in a nucleus as its rest mass energy minus an effective binding energy which decreases as we go to higher energy levels. Eventually this effective binding energy has become zero so that the highest occupied energy level, the Fermi energy
, is equal to the rest mass of a nucleon. At this point adding a nucleon of the same isospin to the nucleus is not possible as the new nucleon would have a negative effective binding energy — i.e. it is more energetically favourable (system will have lowest overall energy) for the nucleon to be created outside the nucleus. This is the particle drip point for that species.
the drip lines are especially useful as limiting boundaries for explosive nucleosynthesis as well as other circumstances with extreme pressure or temperature conditions such as neutron star
s.
es of high energy nucleons which can be captured on seed nuclei
. In these environments, radiative captures
, whether of protons or neutrons, will be much faster than beta-decays, and as astrophysical environments with both large neutron fluxes and high energy protons are unknown at present, the reaction flow will proceed away from beta-stability towards or up to either the neutron or proton drip lines, respectively. However, once a nucleus reaches a drip line, as we have seen, no more nucleons of that species can be added to the particular nucleus, and the nucleus must first undergo a beta-decay before further nucleon captures can occur.
As neutron capture
s can proceed at any energy regime, neutron photodisintegration is unimportant except at higher energies. However, as proton captures are inhibited by the Coulomb barrier, the cross sections for charged-particle reactions at lower energies are greatly suppressed, and in the higher energy regimes where proton captures have a large probability to occur, there is often a competition between proton capture and photodisintegration in explosive hydrogen burning; but because the proton drip line is relatively much closer to the valley of beta-stability than the neutron drip line, nucleosynthesis in some environments may proceed as far as either nucleon drip line.
probably operates very closely to the neutron drip line. Thus, the reaction flow in the r-process is generally assumed to run along the neutron drip line. However, the astrophysical site of the r-process, while widely believed to take place in core-collapse supernovae, is unknown. Furthermore, the neutron drip line is very poorly determined experimentally, and nuclear mass models give various predictions for the precise location of the neutron drip line. In fact, the nuclear physics of extremely neutron-rich matter is a fairly new subject, and already has led to the discovery of the island of inversion
and halo nuclei such as 11Li, which in consequence of a very diffuse neutron skin, has a total radius comparable to that of 208Pb! Thus, although the neutron drip line and the r-process are linked very closely in research, it is an unknown frontier awaiting future research, both from theory and experiment.
in X-ray bursts
runs at the proton drip line except near some photodisintegration waiting points. This includes the nuclei 21Mg, 30S, 34Ar, 38Ca, 56Ni, 60Zn, 64Ge, 68Se,
72Kr, 76Sr, and 80Zr. One obvious nuclear structure pattern that emerges is the importance of pairing, as one notices all the waiting points above are at nuclei with an even number of protons, and all but 21Mg also have an even number of neutrons. However, the waiting points will depend on the assumptions of the X-ray burst model, such as metallicity
, accretion rate, and the hydrodynamics, along with of course the nuclear uncertainties, and as mentioned above, the exact definition of the waiting point may not be consistent from one study to the next. Although there are nuclear uncertainties, compared to other explosive nucleosynthesis processes, the rp-process is quite well experimentally constrained, as, for example, all the above waiting point nuclei have at the least been observed in the laboratory. Thus as the nuclear physics inputs can be found in the literature or data compilations, the Computational Infrastructure for Nuclear Astrophysics allows one to do post-processing calculations on various X-ray burst models, and define for oneself the criteria for the waiting point, as well as alter any nuclear parameters.
While the rp-process in X-ray bursts may have difficulty by-passing the 64Ge waiting point, certainly in X-ray pulsars where the rp-process is stable, the alpha drip line places an upper limit near A=100 on the mass which can be reached through continuous burning; the exact location of the alpha drip line is a present matter under investigation, and 106Te is known to alpha-decay whereas 103Sb is particle-bound. However, it has been shown that if there are episodes of cooling or mixing of previous ashes into the burning zone, material as heavy as 126Xe can be created.
|- style="height:2em;"
| ||+ || ||→ || ||+ ||
|}
As more and more neutrons are created in nuclei the energy levels for neutrons get filled up to an energy level equal to the rest mass of a neutron. At this point any electron penetrating a nucleus will create a neutron which will "drip" out of the nucleus. At this point we have:
And from this point onwards the equation
applies, where pFn is the Fermi momentum of the neutron. As we go deeper into the neutron star the free neutron density increases, and as the Fermi momentum increases with increasing density, the Fermi energy
increases, so that energy levels lower than the top level reach neutron drip and more and more neutrons drip out of nuclei so that we get nuclei in a neutron fluid. Eventually all the neutrons drip out of nuclei and we have reached the neutron fluid interior of the neutron star.
the location of the drip line for many elements with an even number of protons is known, but none past that point are listed in the evaluated nuclear data. There are a few exceptional cases where, due to nuclear pairing, there are some particle-bound species outside the drip line, such as 8B
and 178Au
. One may also note that nearing the magic numbers, the drip line is less understood. A compilation of the known first unbound nuclei beyond the proton drip line is given below, with the number of protons, Z
and the corresponding isotopes, taken from the National Nuclear Data Center.
Nuclear physics
Nuclear physics is the field of physics that studies the building blocks and interactions of atomic nuclei. The most commonly known applications of nuclear physics are nuclear power generation and nuclear weapons technology, but the research has provided application in many fields, including those...
, the boundaries for nuclear particle-stability are conceptualized as drip lines. The nuclear landscape is understood by plotting boxes, each of which represents a unique nuclear species, on a graph with the number of neutrons increasing on the abscissa
Abscissa
In mathematics, abscissa refers to that element of an ordered pair which is plotted on the horizontal axis of a two-dimensional Cartesian coordinate system, as opposed to the ordinate...
and number of protons increasing along the ordinate
Ordinate
In mathematics, ordinate refers to that element of an ordered pair which is plotted on the vertical axis of a two-dimensional Cartesian coordinate system, as opposed to the abscissa...
, which is commonly referred to as the table of nuclides, being to nuclear physics
Nuclear physics
Nuclear physics is the field of physics that studies the building blocks and interactions of atomic nuclei. The most commonly known applications of nuclear physics are nuclear power generation and nuclear weapons technology, but the research has provided application in many fields, including those...
what the more commonly known periodic table of the elements is to chemistry
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
. However, an arbitrary combination of protons and neutrons does not necessarily yield a stable nucleus
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
, and ultimately when continuing to add more of the same type of nucleon
Nucleon
In physics, a nucleon is a collective name for two particles: the neutron and the proton. These are the two constituents of the atomic nucleus. Until the 1960s, the nucleons were thought to be elementary particles...
s to a given nucleus, the newly formed nucleus will essentially undergo immediate decay where a nucleon of the same isospin quantum number
Isospin
In physics, and specifically, particle physics, isospin is a quantum number related to the strong interaction. This term was derived from isotopic spin, but the term is confusing as two isotopes of a nucleus have different numbers of nucleons; in contrast, rotations of isospin maintain the number...
(proton or neutron) is emitted; colloquially the nucleon has 'leaked' or 'dripped' out of the target nucleus, hence giving rise to the term "drip line". The nucleons drip out of such unstable nuclei for the same reason that water drips from a leaking faucet: the droplet, or nucleon in this case, sees a lower potential which is great enough to overcome surface tension in the case of water droplets, and the strong nuclear force in the case of proton emission
Proton emission
Proton emission is a type of radioactive decay in which a proton is ejected from a nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay, in which case the process is known as beta-delayed proton emission, or can occur from the ground state of very...
or alpha decay
Alpha decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less...
. As nucleons are quantized
Quantization (physics)
In physics, quantization is the process of explaining a classical understanding of physical phenomena in terms of a newer understanding known as "quantum mechanics". It is a procedure for constructing a quantum field theory starting from a classical field theory. This is a generalization of the...
, then only integer values
Integer
The integers are formed by the natural numbers together with the negatives of the non-zero natural numbers .They are known as Positive and Negative Integers respectively...
are plotted on the table of isotopes, indicating that the drip line is not linear
Linear
In mathematics, a linear map or function f is a function which satisfies the following two properties:* Additivity : f = f + f...
but instead looks like a step function
Step function
In mathematics, a function on the real numbers is called a step function if it can be written as a finite linear combination of indicator functions of intervals...
up close.
There are drip lines for protons, neutrons, and alpha particles, and these all play important roles in nuclear astrophysics
Nuclear astrophysics
Nuclear astrophysics is an interdisciplinary branch of physics involving close collaboration among researchers in various subfields of nuclear physics and astrophysics, with significant emphasis in areas such as stellar modeling, measurement and theoretical estimation of nuclear reaction rates,...
.
General description
Nuclear existence on the neutron-rich side of stability is limited by the neutron drip line, and on the proton-rich side stability is limited by the proton drip line. When the material has a reasonable balance of protons and neutrons, the total nuclear mass is limited by alpha decay, or the alpha drip line, which connects the proton and neutron drip lines, but is somewhat more confusing to visualize as it also branches down through the center of the chart. These limits exist because of particle decay, whereby an exothermic nuclear transition can occur by the emission of one or more nucleons (not to be confused with particle decayParticle decay
Particle decay is the spontaneous process of one elementary particle transforming into other elementary particles. During this process, an elementary particle becomes a different particle with less mass and an intermediate particle such as W boson in muon decay. The intermediate particle then...
in particle physics
Particle physics
Particle physics is a branch of physics that studies the existence and interactions of particles that are the constituents of what is usually referred to as matter or radiation. In current understanding, particles are excitations of quantum fields and interact following their dynamics...
).
While the concept of nuclear drip lines is very simple in principle because naturally occurring isotopes on Earth do not undergo proton or neutron emission, and to the complexity of the alpha drip line, the terms are not introduced in some undergraduate textbooks on nuclear physics. The idea may become more common place with the advent of radioactive ion beam
Ion beam
An ion beam is a type of charged particle beam consisting of ions. Ion beams have many uses in electronics manufacturing and other industries. A variety of ion beam sources exist, some derived from the mercury vapor thrusters developed by NASA in the 1960s.-Ion beam etching or sputtering:One type...
accelerators in the late 1980s, which are allowing nuclear physicists to really probe the limits of nuclear stability. To understand the concept, one only needs to apply the principle of conservation of energy to nuclear binding energy
Binding energy
Binding energy is the mechanical energy required to disassemble a whole into separate parts. A bound system typically has a lower potential energy than its constituent parts; this is what keeps the system together—often this means that energy is released upon the creation of a bound state...
.
Allowed transitions
When considering whether a specific nuclear transmutation, whether a reaction or a decay, is energetically allowed, one only needs to sum the masses of the initial nucleus/nuclei and subtract from that value the sum of the masses of the out-going particles. If the result, or Q-value, is positive, then the transmutation is allowed, or exothermic because it releases energy, and if the Q-value is negative, then it is endothermic because at least that much energy must be added to the system before the transmutation may proceed. For example, if one wishes to ask if 12C, the most common isotope of carbon, can undergo proton emission to 11B, one finds that about 16 MeV must be added to the system for this process to be allowed. While Q-values can be used to describe any nuclear transmutations, for particle decay, the quantity S, or the particle separation energy, is also used, and it is equivalent to the negative of the Q-value; in other words, the proton separation energy Sp indicates how much energy should be added to a given nucleus to remove a single proton. Thus, the particle drip lines are defined as the boundaries where the particle separation energy is less than or equal to zero, which is when spontaneous emission of that particle is energetically allowed.Nuclei near the drip lines are not common on Earth
Of the three types of naturally occurring radioactivities (α, β, and γ), only alpha decayAlpha decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less...
is a type of particle decay; alpha-decay can be considered either a form of particle decay or, less frequently, a special case of nuclear fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
. The timescale for alpha decay can be much longer than for proton or neutron emission owing to the high Coulomb barrier seen by an alpha-cluster in a nucleus. Although there are no naturally occurring nuclei on Earth which undergo proton emission or neutron emission
Neutron emission
Neutron emission is a type of radioactive decay of atoms containing excess neutrons, in which a neutron is simply ejected from the nucleus. Two examples of isotopes which emit neutrons are helium-5 and beryllium-13...
, such nuclei can be created, for example, in the laboratory with accelerators
Particle accelerator
A particle accelerator is a device that uses electromagnetic fields to propel charged particles to high speeds and to contain them in well-defined beams. An ordinary CRT television set is a simple form of accelerator. There are two basic types: electrostatic and oscillating field accelerators.In...
or naturally in stars
Stellar nucleosynthesis
Stellar nucleosynthesis is the collective term for the nuclear reactions taking place in stars to build the nuclei of the elements heavier than hydrogen. Some small quantity of these reactions also occur on the stellar surface under various circumstances...
. Such particle decays are not commonly known because particle decay is governed by the nuclear strong force
Strong interaction
In particle physics, the strong interaction is one of the four fundamental interactions of nature, the others being electromagnetism, the weak interaction and gravitation. As with the other fundamental interactions, it is a non-contact force...
, as well as the Coulomb force in the case of charged particles, which can act very quickly (femtoseconds or less). In nuclear physics terms, nuclei that are outside the drip lines are particle-unbound and considered not to exist, because they can only exist in the energy continuum rather than in the discrete quantized states we are familiar with. In a discussion of the proton and neutron drip lines, one nomenclatural convenience is to regard beta-unstable nuclei as stable (strictly speaking they are particle-stable), due to the significant difference in the time-scales of these two different decay modes. Thus, the only type of nuclei which are longer lived and undergo proton or neutron emission are in the class of beta-delayed decays, where first the isospin of one nucleon is reversed (proton to neutron or vice versa) via beta-decay, and then if the particle separation energy is non-positive, the daughter nucleus will undergo particle decay. Most naturally occurring γ-sources are technically β-delayed γ-decay, so this concept should be familiar; some gamma-sources are α-delayed but these are generally categorized with other alpha-sources.
Nuclear structure origin of the drip lines
We can see how the drip lines originate by considering the energy levels in a nucleus. The energy of a nucleon in a nucleus is its rest mass energy minus a binding energyBinding energy
Binding energy is the mechanical energy required to disassemble a whole into separate parts. A bound system typically has a lower potential energy than its constituent parts; this is what keeps the system together—often this means that energy is released upon the creation of a bound state...
. In addition to this, however, there is an energy due to degeneracy: for instance a nucleon with energy E1 will be forced to a higher energy E2 if all the lower energy states are filled. This is because nucleons are fermions and obey Fermi-Dirac statistics
Fermi-Dirac statistics
Fermi–Dirac statistics is a part of the science of physics that describes the energies of single particles in a system comprising many identical particles that obey the Pauli Exclusion Principle...
. The work done in putting this nucleon to a higher energy level results in a pressure which is the degeneracy pressure. So we can view the energy of a nucleon in a nucleus as its rest mass energy minus an effective binding energy which decreases as we go to higher energy levels. Eventually this effective binding energy has become zero so that the highest occupied energy level, the Fermi energy
Fermi energy
The Fermi energy is a concept in quantum mechanics usually referring to the energy of the highest occupied quantum state in a system of fermions at absolute zero temperature....
, is equal to the rest mass of a nucleon. At this point adding a nucleon of the same isospin to the nucleus is not possible as the new nucleon would have a negative effective binding energy — i.e. it is more energetically favourable (system will have lowest overall energy) for the nucleon to be created outside the nucleus. This is the particle drip point for that species.
Astrophysical relevance
In nuclear astrophysicsNuclear astrophysics
Nuclear astrophysics is an interdisciplinary branch of physics involving close collaboration among researchers in various subfields of nuclear physics and astrophysics, with significant emphasis in areas such as stellar modeling, measurement and theoretical estimation of nuclear reaction rates,...
the drip lines are especially useful as limiting boundaries for explosive nucleosynthesis as well as other circumstances with extreme pressure or temperature conditions such as neutron star
Neutron star
A neutron star is a type of stellar remnant that can result from the gravitational collapse of a massive star during a Type II, Type Ib or Type Ic supernova event. Such stars are composed almost entirely of neutrons, which are subatomic particles without electrical charge and with a slightly larger...
s.
Nucleosynthesis
Explosive astrophysical environments often have very large fluxFlux
In the various subfields of physics, there exist two common usages of the term flux, both with rigorous mathematical frameworks.* In the study of transport phenomena , flux is defined as flow per unit area, where flow is the movement of some quantity per time...
es of high energy nucleons which can be captured on seed nuclei
Seed nucleus
A seed nucleus is an isotope that is the starting point for any of a variety of fusion chain reactions. The mix of nuclei produced at the conclusion of the chain reaction generally depends strongly on the relative availability of the seed nucleus or nuclei and the component being fused--whether...
. In these environments, radiative captures
Neutron capture
Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled...
, whether of protons or neutrons, will be much faster than beta-decays, and as astrophysical environments with both large neutron fluxes and high energy protons are unknown at present, the reaction flow will proceed away from beta-stability towards or up to either the neutron or proton drip lines, respectively. However, once a nucleus reaches a drip line, as we have seen, no more nucleons of that species can be added to the particular nucleus, and the nucleus must first undergo a beta-decay before further nucleon captures can occur.
Photodisintegration
While the drip lines impose the ultimate boundaries for nucleosynthesis, in high energy environments the burning pathway may be limited before the drip lines by photodisintegration, where a high energy gamma ray knocks a nucleon out of a nucleus. The same nucleus is subject both to a flux of nucleons and photons, so an equilibrium is reached where mass builds up at particular nuclear species. In this sense one might also imagine a similar drip line which applies to photodisintegration in particular environments, but because the nucleons are energetically knocked-out of nuclei and not dripping out in such a case, the terminology is misleading and is not used. As the photon bath will typically be described by a Planckian distribution, higher energy photons will be less abundant, and so photodisintegration will not become significant until the nucleon separation energy begins to approach zero towards the drip lines, where photodisintegration may be induced by lower energy gamma rays. At 1 × 109 Kelvin, the photon distribution is energetic enough to knock nucleons out of any nuclei with particle separation energies less than 3 MeV, but to know which nuclei exist in what abundances one must consider also the competing radiative captures.As neutron capture
Neutron capture
Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled...
s can proceed at any energy regime, neutron photodisintegration is unimportant except at higher energies. However, as proton captures are inhibited by the Coulomb barrier, the cross sections for charged-particle reactions at lower energies are greatly suppressed, and in the higher energy regimes where proton captures have a large probability to occur, there is often a competition between proton capture and photodisintegration in explosive hydrogen burning; but because the proton drip line is relatively much closer to the valley of beta-stability than the neutron drip line, nucleosynthesis in some environments may proceed as far as either nucleon drip line.
Waiting points and time scales
Once radiative capture can no longer proceed on a given nucleus, either from photodisintegration or the drip lines, further nuclear processing to higher mass must either bypass this nucleus by undergoing a reaction with a heavier nucleus such as 4He, or more often wait for the beta decay. Nuclear species where a significant fraction of the mass builds up during a particular nucleosynthesis episode are considered nuclear waiting points, since further processing by fast radiative captures is delayed. There is not an explicit definition of what constitutes a nuclear waiting point, and some quantitative criteria relating the mass fraction at a given nucleus for a given time with respect to the nucleosynthesis time scale is desirable. As has been emphasized, the beta-decays are the slowest processes occurring in explosive nucleosynthesis. From the nuclear physics side, explosive nucleosynthesis time scales are set simply by summing the beta decay half-lives involved, since the time scale for other nuclear processes is negligible in comparison, although practically speaking this time scale is dominated by the sum merely of a handful of waiting point nuclear half lives typically.The r-process
The rapid neutron capture processR-process
The r-process is a nucleosynthesis process, likely occurring in core-collapse supernovae responsible for the creation of approximately half of the neutron-rich atomic nuclei that are heavier than iron. The process entails a succession of rapid neutron captures on seed nuclei, typically Ni-56,...
probably operates very closely to the neutron drip line. Thus, the reaction flow in the r-process is generally assumed to run along the neutron drip line. However, the astrophysical site of the r-process, while widely believed to take place in core-collapse supernovae, is unknown. Furthermore, the neutron drip line is very poorly determined experimentally, and nuclear mass models give various predictions for the precise location of the neutron drip line. In fact, the nuclear physics of extremely neutron-rich matter is a fairly new subject, and already has led to the discovery of the island of inversion
Island of inversion
An island of inversion is a region of the chart of nuclides that contains isotopes with a non-standard ordering of single particle levels in the nuclear shell model. Such an area was first described in 1975 by French physicists carrying out spectroscopic mass measurements of exotic isotopes of...
and halo nuclei such as 11Li, which in consequence of a very diffuse neutron skin, has a total radius comparable to that of 208Pb! Thus, although the neutron drip line and the r-process are linked very closely in research, it is an unknown frontier awaiting future research, both from theory and experiment.
The rp-process
The rapid proton capture processRp-process
The rp-process consists of consecutive proton captures onto seed nuclei to produce heavier elements. It is a nucleosynthesis process and, along with the s process and the r process, may be responsible for the generation of many of the heavy elements present in the universe...
in X-ray bursts
X-ray burster
X-ray bursters are one class of X-ray binary stars exhibiting periodic and rapid increases in luminosity peaked in the X-ray regime of the electromagnetic spectrum...
runs at the proton drip line except near some photodisintegration waiting points. This includes the nuclei 21Mg, 30S, 34Ar, 38Ca, 56Ni, 60Zn, 64Ge, 68Se,
72Kr, 76Sr, and 80Zr. One obvious nuclear structure pattern that emerges is the importance of pairing, as one notices all the waiting points above are at nuclei with an even number of protons, and all but 21Mg also have an even number of neutrons. However, the waiting points will depend on the assumptions of the X-ray burst model, such as metallicity
Metallicity
In astronomy and physical cosmology, the metallicity of an object is the proportion of its matter made up of chemical elements other than hydrogen and helium...
, accretion rate, and the hydrodynamics, along with of course the nuclear uncertainties, and as mentioned above, the exact definition of the waiting point may not be consistent from one study to the next. Although there are nuclear uncertainties, compared to other explosive nucleosynthesis processes, the rp-process is quite well experimentally constrained, as, for example, all the above waiting point nuclei have at the least been observed in the laboratory. Thus as the nuclear physics inputs can be found in the literature or data compilations, the Computational Infrastructure for Nuclear Astrophysics allows one to do post-processing calculations on various X-ray burst models, and define for oneself the criteria for the waiting point, as well as alter any nuclear parameters.
While the rp-process in X-ray bursts may have difficulty by-passing the 64Ge waiting point, certainly in X-ray pulsars where the rp-process is stable, the alpha drip line places an upper limit near A=100 on the mass which can be reached through continuous burning; the exact location of the alpha drip line is a present matter under investigation, and 106Te is known to alpha-decay whereas 103Sb is particle-bound. However, it has been shown that if there are episodes of cooling or mixing of previous ashes into the burning zone, material as heavy as 126Xe can be created.
Neutron stars
In neutron stars, neutron heavy nuclei are found as relativistic electrons penetrate the nuclei and produce inverse beta decay, wherein the electron combines with a proton in the nucleus to make a neutron and an electron-neutrino:- {| border="0"
|- style="height:2em;"
| ||+ || ||→ || ||+ ||
|}
As more and more neutrons are created in nuclei the energy levels for neutrons get filled up to an energy level equal to the rest mass of a neutron. At this point any electron penetrating a nucleus will create a neutron which will "drip" out of the nucleus. At this point we have:
And from this point onwards the equation
applies, where pFn is the Fermi momentum of the neutron. As we go deeper into the neutron star the free neutron density increases, and as the Fermi momentum increases with increasing density, the Fermi energy
Fermi energy
The Fermi energy is a concept in quantum mechanics usually referring to the energy of the highest occupied quantum state in a system of fermions at absolute zero temperature....
increases, so that energy levels lower than the top level reach neutron drip and more and more neutrons drip out of nuclei so that we get nuclei in a neutron fluid. Eventually all the neutrons drip out of nuclei and we have reached the neutron fluid interior of the neutron star.
Neutron drip line
The values of the neutron drip line are only known for the first eight elements, hydrogen to oxygen. For Z = 8, the maximal number of neutrons is 16, resulting in O-24 as the heaviest possible oxygen isotope.Proton drip line
The general location of the proton drip line is well established. For all elements occurring naturally on earth and having an odd number of protons, at least one species with a proton separation energy less than zero has been experimentally observed. Up to germaniumGermanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. The isolated element is a semiconductor, with an appearance most similar to elemental silicon....
the location of the drip line for many elements with an even number of protons is known, but none past that point are listed in the evaluated nuclear data. There are a few exceptional cases where, due to nuclear pairing, there are some particle-bound species outside the drip line, such as 8B
Isotopes of boron
Boron naturally occurs in two isotopes, 10B and 11B, the later of which makes up about 80% of natural boron. 14 radioisotopes have been discovered, with mass numbers from 6 to 21, all with short half-lives, the longest being that of 8B, with a half-life of only 770 ms and 12B with a half-life of...
and 178Au
Isotopes of gold
Gold has one stable isotope, 197Au, and 36 radioisotopes with 195Au being the most stable with a half-life of 186 days.Gold is currently considered the heaviest Monoisotopic element .Gold has been proposed as a material for creating a salted nuclear weapon Gold (Au) has one stable isotope, 197Au,...
. One may also note that nearing the magic numbers, the drip line is less understood. A compilation of the known first unbound nuclei beyond the proton drip line is given below, with the number of protons, Z
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
and the corresponding isotopes, taken from the National Nuclear Data Center.
| Z | Species |
|---|---|
| 1 | N/A |
| 2 | 2He |
| 3 | 5Li |
| 4 | 5Be |
| 5 | 7B, 9B |
| 7 | 11N |
| 8 | 12O |
| 9 | 16F |
| 11 | 19Na |
| 12 | 19Mg |
| 13 | 21Al |
| 15 | 25P |
| 17 | 30Cl |
| 19 | 34K |
| 21 | 39Sc |
| 23 | 42V |
| 25 | 45Mn |
| 27 | 50Co |
| 29 | 55Cu |
| 31 | 59Ga |
| 32 | 58Ge |
| 33 | 65As |
| 35 | 69Br |
| 37 | 73Rb |
| 39 | 77Y |
| 41 | 81Nb |
| 43 | 85Tc |
| 45 | 89Rh |
| 47 | 93Ag |
| 49 | 97In |
| 51 | 105Sb |
| 53 | 110I |
| 55 | 115Cs |
| 57 | 119La |
| 59 | 123Pr |
| 61 | 128Pm |
| 63 | 134Eu |
| 65 | 139Tb |
| 67 | 145Ho |
| 69 | 149Tm |
| 71 | 155Lu |
| 73 | 159Ta |
| 75 | 165Re |
| 77 | 171Ir |
| 79 | 175Au, 177Au |
| 81 | 181Tl |
| 83 | 189Bi |
| 85 | 195At |
| 87 | 201Fr |
| 89 | 207Ac |
| 91 | 214Pa |
See also
- Extension of the periodic table beyond the seventh period
- Period 8 elementPeriod 8 elementA period 8 element is any one of 50 hypothetical chemical elements belonging to an eighth period of the periodic table of the elements. They may be referred to using IUPAC systematic element names. None of these elements have been created, and it is possible that none have isotopes with stable...
- Table of nuclidesTable of nuclidesThe tables listed below provide information on the basic properties of all nuclides.* Neutron + Element 1 - Element 24 * Element 25 - Element 48 * Element 49 - Element 72...
- Radioactive decayRadioactive decayRadioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...