
R-process
Encyclopedia
The r-process is a nucleosynthesis
process, likely occurring in core-collapse supernova
e (see also supernova nucleosynthesis
) responsible for the creation of approximately half of the neutron
-rich atomic nuclei
that are heavier than iron
. The process entails a succession of rapid neutron capture
s on seed nuclei
, typically Ni-56, hence the name r-process. The other predominant mechanism for the production of heavy elements is the s-process
, which is nucleosynthesis by means of slow neutron captures, primarily occurring in AGB stars
, and together these two processes account for a majority of galactic chemical evolution
of elements heavier than iron.
by Hans Suess
and Harold Urey
in 1956. Among other things, these data show abundance peaks for germanium
, xenon
, and platinum
. According to quantum mechanics
and the nuclear shell model
, radioactive nuclei that decay into isotopes of these elements have closed neutron shells near the neutron drip line. This implies that some abundant nuclei must be created by rapid neutron capture
, and it was only a matter of determining what other nuclei could be accounted for by such a process. A table apportioning the heavy isotopes between s-process
and r-process was published in a famous review paper in 1957, which proposed the theory of stellar nucleosynthesis
and set the frame-work for contemporary nuclear astrophysics
.
(on the order of 1022 neutrons per cm2 per second) and temperature
, so that neutron capture
s occur much faster than beta-minus decays
far from stability, meaning that the r-process "runs up" along the neutron drip line. The only hold-ups inhibiting this process of climbing the neutron drip line are a notable decrease in the neutron-capture cross section
at nuclei with closed neutron shells, the competing photodisintegration [(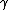 ,n)] reaction rates, and the degree of nuclear stability in the heavy-isotope region, which terminates the r-process when such nuclei become readily unstable to spontaneous fission (currently believed to be in the neutron-rich region near A = 270 (number of nucleons) in the chart of nuclides
,n)] reaction rates, and the degree of nuclear stability in the heavy-isotope region, which terminates the r-process when such nuclei become readily unstable to spontaneous fission (currently believed to be in the neutron-rich region near A = 270 (number of nucleons) in the chart of nuclides
). After the neutron flux decreases, these highly unstable radioactive
nuclei quickly decay to form stable, neutron-rich nuclei. So, while the s-process
creates an abundance of stable nuclei with closed neutron shells, the r-process creates an abundance of nuclei about 10 amu
below the s-process peaks, as the r-process nuclei decay back towards stability on a constant A line in the chart of nuclides.
e (spectral Type Ib, Ic and II), which provide the necessary physical conditions for the R-process. However, the abundance of r-process nuclei
requires that either only a small fraction of supernovae eject r-process nuclei to the interstellar medium
, or that each supernova ejects only a very small amount of r-process material. A recently proposedalternative solution is that neutron star
mergers (a binary star system
of two neutron stars that collide) may also play a role in the production of r-process nuclei, but this has yet to be observationally
confirmed.
Nucleosynthesis
Nucleosynthesis is the process of creating new atomic nuclei from pre-existing nucleons . It is thought that the primordial nucleons themselves were formed from the quark–gluon plasma from the Big Bang as it cooled below two trillion degrees...
process, likely occurring in core-collapse supernova
Supernova
A supernova is a stellar explosion that is more energetic than a nova. It is pronounced with the plural supernovae or supernovas. Supernovae are extremely luminous and cause a burst of radiation that often briefly outshines an entire galaxy, before fading from view over several weeks or months...
e (see also supernova nucleosynthesis
Supernova nucleosynthesis
Supernova nucleosynthesis is the production of new chemical elements inside supernovae. It occurs primarily due to explosive nucleosynthesis during explosive oxygen burning and silicon burning...
) responsible for the creation of approximately half of the neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
-rich atomic nuclei
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
that are heavier than iron
Heavy metals
A heavy metal is a member of a loosely-defined subset of elements that exhibit metallic properties. It mainly includes the transition metals, some metalloids, lanthanides, and actinides. Many different definitions have been proposed—some based on density, some on atomic number or atomic weight,...
. The process entails a succession of rapid neutron capture
Neutron capture
Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled...
s on seed nuclei
Seed nucleus
A seed nucleus is an isotope that is the starting point for any of a variety of fusion chain reactions. The mix of nuclei produced at the conclusion of the chain reaction generally depends strongly on the relative availability of the seed nucleus or nuclei and the component being fused--whether...
, typically Ni-56, hence the name r-process. The other predominant mechanism for the production of heavy elements is the s-process
S-process
The S-process or slow-neutron-capture-process is a nucleosynthesis process that occurs at relatively low neutron density and intermediate temperature conditions in stars. Under these conditions the rate of neutron capture by atomic nuclei is slow relative to the rate of radioactive beta-minus decay...
, which is nucleosynthesis by means of slow neutron captures, primarily occurring in AGB stars
Asymptotic Giant Branch
The asymptotic giant branch is the region of the Hertzsprung-Russell diagram populated by evolving low to medium-mass stars. This is a period of stellar evolution undertaken by all low to intermediate mass stars late in their lives....
, and together these two processes account for a majority of galactic chemical evolution
Chemical evolution
Chemical evolution may refer to:*Nucleosynthesis, the creation of chemical elements in the universe either through the Big Bang, or supernovae*Abiogenesis, the transition from nonliving elements to living systems...
of elements heavier than iron.
History
The r-process was seen to be needed from the relative abundances of isotopes of heavy elements and from a newly published table of abundancesAbundance of the chemical elements
The abundance of a chemical element measures how relatively common the element is, or how much of the element is present in a given environment by comparison to all other elements...
by Hans Suess
Hans Suess
Hans Eduard Suess was an Austrian physical chemist and nuclear physicist. He was a grandson of the Austrian geologist Eduard SuessSuess earned his Ph.D. in chemistry from the University of Vienna in 1935...
and Harold Urey
Harold Urey
Harold Clayton Urey was an American physical chemist whose pioneering work on isotopes earned him the Nobel Prize in Chemistry in 1934...
in 1956. Among other things, these data show abundance peaks for germanium
Germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. The isolated element is a semiconductor, with an appearance most similar to elemental silicon....
, xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
, and platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
. According to quantum mechanics
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
and the nuclear shell model
Shell model
In nuclear physics and nuclear chemistry, the nuclear shell model is a model of the atomic nucleus which uses the Pauli exclusion principle to describe the structure of the nucleus in terms of energy levels. The first shell model was proposed by Dmitry Ivanenko in 1932...
, radioactive nuclei that decay into isotopes of these elements have closed neutron shells near the neutron drip line. This implies that some abundant nuclei must be created by rapid neutron capture
Neutron capture
Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled...
, and it was only a matter of determining what other nuclei could be accounted for by such a process. A table apportioning the heavy isotopes between s-process
S-process
The S-process or slow-neutron-capture-process is a nucleosynthesis process that occurs at relatively low neutron density and intermediate temperature conditions in stars. Under these conditions the rate of neutron capture by atomic nuclei is slow relative to the rate of radioactive beta-minus decay...
and r-process was published in a famous review paper in 1957, which proposed the theory of stellar nucleosynthesis
Stellar nucleosynthesis
Stellar nucleosynthesis is the collective term for the nuclear reactions taking place in stars to build the nuclei of the elements heavier than hydrogen. Some small quantity of these reactions also occur on the stellar surface under various circumstances...
and set the frame-work for contemporary nuclear astrophysics
Nuclear astrophysics
Nuclear astrophysics is an interdisciplinary branch of physics involving close collaboration among researchers in various subfields of nuclear physics and astrophysics, with significant emphasis in areas such as stellar modeling, measurement and theoretical estimation of nuclear reaction rates,...
.
Nuclear physics
Immediately after a core-collapse supernova, there is an extremely high neutron fluxNeutron flux
The neutron flux is a quantity used in reactor physics corresponding to the total length travelled by all neutrons per unit time and volume . The neutron fluence is defined as the neutron flux integrated over a certain time period....
(on the order of 1022 neutrons per cm2 per second) and temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
, so that neutron capture
Neutron capture
Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled...
s occur much faster than beta-minus decays
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
far from stability, meaning that the r-process "runs up" along the neutron drip line. The only hold-ups inhibiting this process of climbing the neutron drip line are a notable decrease in the neutron-capture cross section
Nuclear cross section
The nuclear cross section of a nucleus is used to characterize the probability that a nuclear reaction will occur. The concept of a nuclear cross section can be quantified physically in terms of "characteristic area" where a larger area means a larger probability of interaction...
at nuclei with closed neutron shells, the competing photodisintegration [(
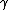 ,n)] reaction rates, and the degree of nuclear stability in the heavy-isotope region, which terminates the r-process when such nuclei become readily unstable to spontaneous fission (currently believed to be in the neutron-rich region near A = 270 (number of nucleons) in the chart of nuclides
,n)] reaction rates, and the degree of nuclear stability in the heavy-isotope region, which terminates the r-process when such nuclei become readily unstable to spontaneous fission (currently believed to be in the neutron-rich region near A = 270 (number of nucleons) in the chart of nuclidesTable of nuclides
The tables listed below provide information on the basic properties of all nuclides.* Neutron + Element 1 - Element 24 * Element 25 - Element 48 * Element 49 - Element 72...
). After the neutron flux decreases, these highly unstable radioactive
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
nuclei quickly decay to form stable, neutron-rich nuclei. So, while the s-process
S-process
The S-process or slow-neutron-capture-process is a nucleosynthesis process that occurs at relatively low neutron density and intermediate temperature conditions in stars. Under these conditions the rate of neutron capture by atomic nuclei is slow relative to the rate of radioactive beta-minus decay...
creates an abundance of stable nuclei with closed neutron shells, the r-process creates an abundance of nuclei about 10 amu
Atomic mass unit
The unified atomic mass unit or dalton is a unit that is used for indicating mass on an atomic or molecular scale. It is defined as one twelfth of the rest mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state, and has a value of...
below the s-process peaks, as the r-process nuclei decay back towards stability on a constant A line in the chart of nuclides.
Astrophysical sites
The most widely believed candidate site for the r-process are core-collapse supernovaSupernova
A supernova is a stellar explosion that is more energetic than a nova. It is pronounced with the plural supernovae or supernovas. Supernovae are extremely luminous and cause a burst of radiation that often briefly outshines an entire galaxy, before fading from view over several weeks or months...
e (spectral Type Ib, Ic and II), which provide the necessary physical conditions for the R-process. However, the abundance of r-process nuclei
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
requires that either only a small fraction of supernovae eject r-process nuclei to the interstellar medium
Interstellar medium
In astronomy, the interstellar medium is the matter that exists in the space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, dust, and cosmic rays. It fills interstellar space and blends smoothly into the surrounding intergalactic space...
, or that each supernova ejects only a very small amount of r-process material. A recently proposedalternative solution is that neutron star
Neutron star
A neutron star is a type of stellar remnant that can result from the gravitational collapse of a massive star during a Type II, Type Ib or Type Ic supernova event. Such stars are composed almost entirely of neutrons, which are subatomic particles without electrical charge and with a slightly larger...
mergers (a binary star system
Binary star
A binary star is a star system consisting of two stars orbiting around their common center of mass. The brighter star is called the primary and the other is its companion star, comes, or secondary...
of two neutron stars that collide) may also play a role in the production of r-process nuclei, but this has yet to be observationally
Observational astronomy
Observational astronomy is a division of the astronomical science that is concerned with getting data, in contrast with theoretical astrophysics which is mainly concerned with finding out the measurable implications of physical models...
confirmed.

