
Nitric oxide dioxygenase
Encyclopedia
Nitric oxide dioxygenase is an enzyme
that catalyzes the conversion of toxic nitric oxide
(NO) to nitrate
(NO). The net reaction for the reaction catalyzed by nitric oxide dioxygenase is shown below:
Nitric oxide is a ubiquitous small molecule that is integrated in a wide variety of physiological processes including smooth muscle vasodilation, platelet disaggregation, neurotransmission, and immune response to bacterial infection. Overproduction of this signaling molecule can be lethal to cells by poisoning cellular energy production. The most sensitive targets of NO are aconitase
, an enzyme that catalyzes the isomerization of citrate
to isocitrate in the citric acid cycle, and cytochrome oxidase, the last enzyme in the respiratory electron transport chain of mitochondria. Additionally NO, with its lone radical on the nitrogen atom, is implicated in a number of secondary mechanisms of toxicity, including catalase
inhibition (resulting in hydrogen peroxide toxicity), Fe-S center iron liberation, and the formation of dinitosyl-iron complexes.
Due to the potential lethality of NO, cells benefitted greatly from the evolution of an enzyme capable of catalyzing the conversion of toxic NO to nitrate.
A 'nitric oxide dioxygenase' is an enzyme that is capable of carrying out this reaction. NO dioxygenase belongs to the family of oxidoreductase
s, more specifically those acting on paired donors, with O2 as oxidant and with incorporation of two atoms of oxygen into the other donor.
Another theory developed more recently (2009) suggests that a NO dioxygenase activity could also proceed through phenolic nitration via a putative heme-peroxynitrite intermediate.
The most well studied NO dioxygenase is flavohemoglobin (flavoHb), shown to the right:
Studies have shown that flavohemoglobins are induced by NO, nitrite, nitrate, and NO-releasing agents in various bacteria and fungi. Additionally, flavoHbs have been shown to protect bacteria, yeast, and Dictyostelium discoideum against growth inhibition and damage mediated via NO.
toxicity. The enzyme was identified with the E. coli
flavohemoglobin.
More recently, another protein has been identified as a NO dioxygenase - rhodobacter sphaeroides haem protein (SHP), a novel cytochrome with NO dioxygenase activity. Although the biological function of SHP has yet to be identified, SHP has been shown, that with oxygen bound, it can react rapidly with nitric oxide to form nitrate.
, and a b-type heme-containing "globin
" domain and optionally an oxidoreductase NAD-binding domain
. The reductase domain supplies an electron to the heme iron to achieve a high rate of catalytic NO dioxygenation.
In addition to numerous flavohemoglobins, many distantly related members of the hemoglobin
superfamily including the muscle myoglobin
, the non-symbiotic plant hemoglobin and symbiotic plant leghemoglobin
, the neuronal neuroglobin
, and the mammalian cytoplasmic cytoglobin
appear to function as nitric oxide dioxygenases (NODs), although the cellular electron donor(s) for many globins have yet to be defined. Electron donors may include ascorbate, cytochrome b5 or ferredoxin reductase. The catalytic NO dioxygenation can be written in its simplest form:
Catalysis is very efficient. The reported bimolecular NO dioxygenation rate constants range from 2 x 107 M−1s−1 for cytoglobin to 3 x 109 M−1s−1 for flavohemoglobin, and turnover rates range from 1 to 700 s−1. Structure, O2 binding, and reduction of globins appear optimized for a NO dioxygenase function.
The wide diversity of multicellular organisms benefitting from the oxygen storage and transport functions of myoglobin/hemoglobin appeared much later (approximately 0.5 billion years ago).
NODs are now known to serve two important physiological functions in diverse life forms. They prevent NO toxicity and regulate NO signalling. NODs belong to the larger family of well-established free radical and reactive oxygen detoxifying enzymes that includes superoxide dismutase
, catalase
, and peroxidase
.
which was first investigated and reported over a century earlier by Felix Hoppe-Seyler and others. Other proteins that may act as NODs include mammalian microsomal cytochrome P450(s) and a novel O2-binding cytochrome b from Rhodobacter sphaeroides
.
antibiotics. Imidazoles have been shown to coordinate with the heme iron atom of microbial flavohemoglobin, impair ferric heme reduction, produce uncompetitive inhibition with respect to O2 and NO, and inhibit NO metabolism by yeasts and bacteria. Specifically, imidazoles bearing bulky aromatic substituents have been shown to have potential for selective and high-affinity inhibition of NO dioxygenase function by coordinating the catalytic heme iron and "fitting" within the large hydrophobic distal heme pocket. As a result, imidazole engineering has been suggested as a means to specifically inhibit NO dioxygenases.
In addition, genetically modified plants with heterologous flavohemoglobin-NODs are being developed to limit NO toxicity created by metabolism of nitrogen fertilizers by soil microbes and as a means towards plant self-fertilization through absorption of environmental NO.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes the conversion of toxic nitric oxide
Nitric oxide
Nitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
(NO) to nitrate
Nitrate
The nitrate ion is a polyatomic ion with the molecular formula NO and a molecular mass of 62.0049 g/mol. It is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically-bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a...
(NO). The net reaction for the reaction catalyzed by nitric oxide dioxygenase is shown below:
- 2NO + 2O2 + NAD(P)HNicotinamide adenine dinucleotide phosphateNicotinamide adenine dinucleotide phosphate, abbreviated NADP or TPN in older notation , is a coenzyme used in anabolic reactions, such as lipid and nucleic acid synthesis, which require NADPH as a reducing agent....
→ 2NO3- + NAD(P)+ + H+
Nitric oxide is a ubiquitous small molecule that is integrated in a wide variety of physiological processes including smooth muscle vasodilation, platelet disaggregation, neurotransmission, and immune response to bacterial infection. Overproduction of this signaling molecule can be lethal to cells by poisoning cellular energy production. The most sensitive targets of NO are aconitase
Aconitase
Aconitase is an enzyme that catalyses the stereo-specific isomerization of citrate to isocitrate via cis-aconitate in the tricarboxylic acid cycle, a non-redox-active process.- Function :...
, an enzyme that catalyzes the isomerization of citrate
Citrate
A citrate can refer either to the conjugate base of citric acid, , or to the esters of citric acid. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate.-Other citric acid ions:...
to isocitrate in the citric acid cycle, and cytochrome oxidase, the last enzyme in the respiratory electron transport chain of mitochondria. Additionally NO, with its lone radical on the nitrogen atom, is implicated in a number of secondary mechanisms of toxicity, including catalase
Catalase
Catalase is a common enzyme found in nearly all living organisms that are exposed to oxygen, where it catalyzes the decomposition of hydrogen peroxide to water and oxygen...
inhibition (resulting in hydrogen peroxide toxicity), Fe-S center iron liberation, and the formation of dinitosyl-iron complexes.
Due to the potential lethality of NO, cells benefitted greatly from the evolution of an enzyme capable of catalyzing the conversion of toxic NO to nitrate.
A 'nitric oxide dioxygenase' is an enzyme that is capable of carrying out this reaction. NO dioxygenase belongs to the family of oxidoreductase
Oxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule to another...
s, more specifically those acting on paired donors, with O2 as oxidant and with incorporation of two atoms of oxygen into the other donor.
Reaction mechanism
The mechanism of action has still not been entirely deduced, however, the leading theory suggests that the conversion is carried out through a series of redox reactions involving iron centers as shown in the series of half reactions below:| Step | Reaction |
|---|---|
| FAD reduction | NAD(P)H + FAD + H+ → NAD(P)+ + FADH2 |
| Iron reduction 1 | FADH2 + Fe3+ → Fe2+ + FADH + H+ |
| Iron Reduction 2 | FADH + Fe3+ → FAD + Fe2+ + H+ |
| O2 Binding | Fe2+ + O2 → Fe3+(O2-) |
| NO dioxygenation | Fe3+(O2-) + NO → Fe3+ + NO3- |
Another theory developed more recently (2009) suggests that a NO dioxygenase activity could also proceed through phenolic nitration via a putative heme-peroxynitrite intermediate.
The most well studied NO dioxygenase is flavohemoglobin (flavoHb), shown to the right:
Studies have shown that flavohemoglobins are induced by NO, nitrite, nitrate, and NO-releasing agents in various bacteria and fungi. Additionally, flavoHbs have been shown to protect bacteria, yeast, and Dictyostelium discoideum against growth inhibition and damage mediated via NO.
Discovery
Nitric oxide dioxygenase was discovered, and first reported in 1998, as an inducible O2-dependent enzymatic activity that protected bacteria against nitric oxideNitric oxide
Nitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
toxicity. The enzyme was identified with the E. coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
flavohemoglobin.
More recently, another protein has been identified as a NO dioxygenase - rhodobacter sphaeroides haem protein (SHP), a novel cytochrome with NO dioxygenase activity. Although the biological function of SHP has yet to be identified, SHP has been shown, that with oxygen bound, it can react rapidly with nitric oxide to form nitrate.
Structure and molecular function
The flavohemoglobin protein contains two domains: an oxidoreductase FAD-binding domainOxidoreductase FAD-binding domain
The oxidoreductase FAD-binding domain is an evolutionary conserved protein domain.To date, the 3D-structures of the flavoprotein domain of Zea mays nitrate reductase and of pig NADH:cytochrome b5 reductase have been solved...
, and a b-type heme-containing "globin
Globin
Globins are a related family of proteins, which are thought to share a common ancestor. These proteins all incorporate the globin fold, a series of eight alpha helical segments. Two prominent members of this family include myoglobin and hemoglobin, which both bind the heme prosthetic group...
" domain and optionally an oxidoreductase NAD-binding domain
Oxidoreductase NAD-binding domain
Oxidoreductase NAD-binding domain is an evolutionary conserved protein domain.Xanthine dehydrogenases, that also bind FAD/NAD, have essentially no similarity....
. The reductase domain supplies an electron to the heme iron to achieve a high rate of catalytic NO dioxygenation.
In addition to numerous flavohemoglobins, many distantly related members of the hemoglobin
Hemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
superfamily including the muscle myoglobin
Myoglobin
Myoglobin is an iron- and oxygen-binding protein found in the muscle tissue of vertebrates in general and in almost all mammals. It is related to hemoglobin, which is the iron- and oxygen-binding protein in blood, specifically in the red blood cells. The only time myoglobin is found in the...
, the non-symbiotic plant hemoglobin and symbiotic plant leghemoglobin
Leghemoglobin
Leghemoglobin is a nitrogen or oxygen carrier, because naturally occurring oxygen and nitrogen interact similarly with this protein; and a hemoprotein found in the nitrogen-fixing root nodules of leguminous plants. But nitrogen is necessary for the cycle to occur...
, the neuronal neuroglobin
Neuroglobin
Neuroglobin is a member of the vertebrate globin family involved in cellular oxygen homeostasis. It is an intracellular hemoprotein expressed in the central and peripheral nervous system, cerebrospinal fluid, retina and endocrine tissues. Neuroglobin is a monomer that reversibly binds oxygen with...
, and the mammalian cytoplasmic cytoglobin
Cytoglobin
Cytoglobin is the protein product of CYGB, a human and mammalian gene.Cytoglobin is a globin molecule located in the brain and most notably utilized in marine mammals. It is thought to be a method of protection under conditions of hypoxia. The predicted function of cytoglobin is the transfer of...
appear to function as nitric oxide dioxygenases (NODs), although the cellular electron donor(s) for many globins have yet to be defined. Electron donors may include ascorbate, cytochrome b5 or ferredoxin reductase. The catalytic NO dioxygenation can be written in its simplest form:
- NO + O2 + e-
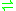 NO3-
NO3-
Catalysis is very efficient. The reported bimolecular NO dioxygenation rate constants range from 2 x 107 M−1s−1 for cytoglobin to 3 x 109 M−1s−1 for flavohemoglobin, and turnover rates range from 1 to 700 s−1. Structure, O2 binding, and reduction of globins appear optimized for a NO dioxygenase function.
Physiological function
Historically, nitric oxide dioxygenase (around 1.8 billion years ago) served to provide the modern day analogue of hemoglobin/myoglobin function for oxygen storage and transport. Gardner et al. (1998) suggested that the first hemoglobin/myoglobin probably functioned as an enzyme utilizing bound ‘activated’ oxygen gas to dioxygenate NO in microbes.The wide diversity of multicellular organisms benefitting from the oxygen storage and transport functions of myoglobin/hemoglobin appeared much later (approximately 0.5 billion years ago).
NODs are now known to serve two important physiological functions in diverse life forms. They prevent NO toxicity and regulate NO signalling. NODs belong to the larger family of well-established free radical and reactive oxygen detoxifying enzymes that includes superoxide dismutase
Superoxide dismutase
Superoxide dismutases are a class of enzymes that catalyze the dismutation of superoxide into oxygen and hydrogen peroxide. As such, they are an important antioxidant defense in nearly all cells exposed to oxygen...
, catalase
Catalase
Catalase is a common enzyme found in nearly all living organisms that are exposed to oxygen, where it catalyzes the decomposition of hydrogen peroxide to water and oxygen...
, and peroxidase
Peroxidase
Peroxidases are a large family of enzymes that typically catalyze a reaction of the form:For many of these enzymes the optimal substrate is hydrogen peroxide, but others are more active with organic hydroperoxides such as lipid peroxides...
.
Distribution in nature
NODs, as well as many hemoglobins that function as NODs, are distributed to most life forms including bacteria, fungi, protists, worms, plants and animals. In fact, nitric oxide dioxygenation appears to be a primal function for members of the hemoglobin superfamily. Moreover, it is becoming increasingly evident that the NOD function of globins is much more common than the paradigmatic O2 transport-storage function of red cell hemoglobinHemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
which was first investigated and reported over a century earlier by Felix Hoppe-Seyler and others. Other proteins that may act as NODs include mammalian microsomal cytochrome P450(s) and a novel O2-binding cytochrome b from Rhodobacter sphaeroides
Rhodobacter sphaeroides
Rhodobacter sphaeroides is a kind of purple bacteria; a group of bacteria that can obtain energy through photosynthesis. Its best growth conditions are anaerobic phototrophy and aerobic chemoheterotrophy in the absence of light. R. sphaeroides is also able to fix nitrogen...
.
Technologies
Inhibitors of the NODs are being developed for application as microbial antibiotics, anti-tumor agents and modulators of NO signalling. The most prominent class of inhibitor of NO dioxygenase to date is imidazoleImidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
antibiotics. Imidazoles have been shown to coordinate with the heme iron atom of microbial flavohemoglobin, impair ferric heme reduction, produce uncompetitive inhibition with respect to O2 and NO, and inhibit NO metabolism by yeasts and bacteria. Specifically, imidazoles bearing bulky aromatic substituents have been shown to have potential for selective and high-affinity inhibition of NO dioxygenase function by coordinating the catalytic heme iron and "fitting" within the large hydrophobic distal heme pocket. As a result, imidazole engineering has been suggested as a means to specifically inhibit NO dioxygenases.
In addition, genetically modified plants with heterologous flavohemoglobin-NODs are being developed to limit NO toxicity created by metabolism of nitrogen fertilizers by soil microbes and as a means towards plant self-fertilization through absorption of environmental NO.

