
Neutron source
Encyclopedia
A Neutron source is a device that emits neutrons. There is a wide variety of different sources, ranging from hand-held radioactive sources to neutron research facilities operating research reactor
s and spallation
sources. Depending upon neutron energy, neutron flux, size of the source, costs, and government regulations, these devices find use in a diverse array of applications in areas of physics, engineering, medicine, nuclear weapons, petroleum exploration, biology, chemistry, nuclear power and other industries.
Radioisotopes which decay with alpha particles packed in a low-Z elemental matrix
Radioisotopes which decay with high energy photons co-located with beryllium or deuterium
Sealed tube neutron generators
Light ion accelerators
High energy photoneutron/photofission
systems
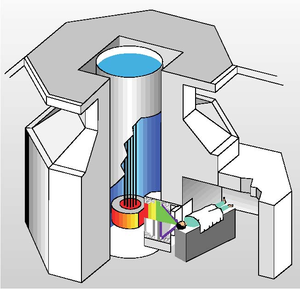 Modern neutron research facilities operate either fission reactor or a spallation source.
Modern neutron research facilities operate either fission reactor or a spallation source.
Nuclear fission reactors
Spallation at particle accelerators
Nuclear fusion systems
is always better (since it reduces the time required to conduct the experiment, acquire the image, etc.). Amateur fusion devices, like the fusor
, generate only about 300 000 neutrons per second. Commercial fusor devices can generate on the order of 109 neutrons per second, which corresponds to a usable flux of less than 105 n/(cm² s). Large neutron beamlines around the world achieve much greater flux. Reactor-based sources now produce 1015 n/(cm² s), and spallation sources generate greater than 1017 n/(cm² s).
Research reactor
Research reactors are nuclear reactors that serve primarily as a neutron source. They are also called non-power reactors, in contrast to power reactors that are used for electricity production, heat generation, or maritime propulsion.-Purpose:...
s and spallation
Spallation
In general, spallation is a process in which fragments of material are ejected from a body due to impact or stress. In the context of impact mechanics it describes ejection or vaporization of material from a target during impact by a projectile...
sources. Depending upon neutron energy, neutron flux, size of the source, costs, and government regulations, these devices find use in a diverse array of applications in areas of physics, engineering, medicine, nuclear weapons, petroleum exploration, biology, chemistry, nuclear power and other industries.
Small devices
Radioisotopes which undergo spontaneous fission- Certain isotopeIsotopeIsotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s undergo spontaneous fissionSpontaneous fissionSpontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
with emission of neutrons. The most commonly used spontaneous fission source is the radioactive isotope californiumCaliforniumCalifornium is a radioactive metallic chemical element with the symbol Cf and atomic number 98. The element was first made in the laboratory in 1950 by bombarding curium with alpha particles at the University of California, Berkeley. It is the ninth member of the actinide series and was the...
-252. Cf-252 and all other spontaneous fission neutron sources are produced by irradiating uraniumUraniumUranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
or another transuranic element in a nuclear reactor, where neutrons are absorbed in the starting material and its subsequent reaction products, transmuting the starting material into the SF isotope. Cf-252 neutron sources are typically 1/4" to 1/2" in diameter and 1" to 2" in length. When purchased new a typical Cf-252 neutron sources emit between 1×107 to 1×109 neutrons per second but, with a half life of 2.6 years, this neutron output rate drops to half of this original value in 2.6 years. The price of a typical Cf-252 neutron source is from $15,000 to $20,000.
Radioisotopes which decay with alpha particles packed in a low-Z elemental matrix
- Neutrons are produced when alpha particleAlpha particleAlpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is classically produced in the process of alpha decay, but may be produced also in other ways and given the same name...
s impinge upon any of several low atomic weight isotopes including isotopes of lithiumLithiumLithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
, berylliumBerylliumBeryllium is the chemical element with the symbol Be and atomic number 4. It is a divalent element which occurs naturally only in combination with other elements in minerals. Notable gemstones which contain beryllium include beryl and chrysoberyl...
, carbonCarbonCarbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
and oxygenOxygenOxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
. This nuclear reaction can be used to construct a neutron source by intermixing a radioisotope that emits alpha particles such as radiumRadiumRadium is a chemical element with atomic number 88, represented by the symbol Ra. Radium is an almost pure-white alkaline earth metal, but it readily oxidizes on exposure to air, becoming black in color. All isotopes of radium are highly radioactive, with the most stable isotope being radium-226,...
or poloniumPoloniumPolonium is a chemical element with the symbol Po and atomic number 84, discovered in 1898 by Marie Skłodowska-Curie and Pierre Curie. A rare and highly radioactive element, polonium is chemically similar to bismuth and tellurium, and it occurs in uranium ores. Polonium has been studied for...
with a low atomic weight isotope, usually in the form of a mixture of powders of the two materials. Typical emission rates for alpha reaction neutron sources range from 1×106 to 1×108 neutrons per second. As an example, a representative alpha-beryllium neutron source can be expected to produce approximately 30 neutrons for every one million alpha particles. The useful lifetime for these types of sources is highly variable, depending upon the half-lifeHalf-lifeHalf-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of the radioisotope that emits the alpha particles. The size and cost of these neutron sources are also comparable to spontaneous fission sources. Usual combinations of materials are plutoniumPlutoniumPlutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
-berylliumBerylliumBeryllium is the chemical element with the symbol Be and atomic number 4. It is a divalent element which occurs naturally only in combination with other elements in minerals. Notable gemstones which contain beryllium include beryl and chrysoberyl...
(PuBe), americiumAmericiumAmericium is a synthetic element that has the symbol Am and atomic number 95. This transuranic element of the actinide series is located in the periodic table below the lanthanide element europium, and thus by analogy was named after another continent, America.Americium was first produced in 1944...
-beryllium (AmBe), or americium-lithiumLithiumLithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
(AmLi). The neutron initiatorsUrchin (detonator)A modulated neutron initiator is a neutron source capable of producing a burst of neutrons on activation. It is a crucial part of some nuclear weapons, as its role is to "kick-start" the chain reaction at the optimal moment when the configuration is prompt critical. It is also known as an internal...
of early nuclear weaponNuclear weaponA nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission or a combination of fission and fusion. Both reactions release vast quantities of energy from relatively small amounts of matter. The first fission bomb test released the same amount...
s used a polonium-beryllium layers separated by nickel and gold until a neutron pulse was desired.
Radioisotopes which decay with high energy photons co-located with beryllium or deuterium
- Gamma radiation with an energy exceeding the neutron binding energyBinding energyBinding energy is the mechanical energy required to disassemble a whole into separate parts. A bound system typically has a lower potential energy than its constituent parts; this is what keeps the system together—often this means that energy is released upon the creation of a bound state...
of a nucleus can eject a neutron. Two examples and their decay products:- 9BeBerylliumBeryllium is the chemical element with the symbol Be and atomic number 4. It is a divalent element which occurs naturally only in combination with other elements in minerals. Notable gemstones which contain beryllium include beryl and chrysoberyl...
+ >1.7 Mev photon → 1 neutron + 2 4He - 2HHydrogenHydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
(deuteriumDeuteriumDeuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
) + >2.26 MeV photon → 1 neutron + 1H
- 9Be
Sealed tube neutron generators
- Some particle acceleratorParticle acceleratorA particle accelerator is a device that uses electromagnetic fields to propel charged particles to high speeds and to contain them in well-defined beams. An ordinary CRT television set is a simple form of accelerator. There are two basic types: electrostatic and oscillating field accelerators.In...
-based neutron generatorNeutron generatorNeutron generators are neutron source devices which contain compact linear accelerators and that produce neutrons by fusing isotopes of hydrogen together. The fusion reactions take place in these devices by accelerating either deuterium, tritium, or a mixture of these two isotopes into a metal...
s exist that work by inducing nuclear fusionNuclear fusionNuclear fusion is the process by which two or more atomic nuclei join together, or "fuse", to form a single heavier nucleus. This is usually accompanied by the release or absorption of large quantities of energy...
between beams of deuteriumDeuteriumDeuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
and/or tritiumTritiumTritium is a radioactive isotope of hydrogen. The nucleus of tritium contains one proton and two neutrons, whereas the nucleus of protium contains one proton and no neutrons...
ions and metal hydride targets which also contain these isotopes.
Moderately-sized devices
Plasma focus and plasma pinch devices- The plasmaPlasma (physics)In physics and chemistry, plasma is a state of matter similar to gas in which a certain portion of the particles are ionized. Heating a gas may ionize its molecules or atoms , thus turning it into a plasma, which contains charged particles: positive ions and negative electrons or ions...
focus neutron source (see dense plasma focusDense plasma focusA dense plasma focus is a machine that produces, by electromagnetic acceleration and compression, a short-lived plasma that is so hot and dense that it can cause nuclear fusion and emit X-rays. The electromagnetic compression of the plasma is called a pinch. It was invented in the early 1960s by...
, not to be confused with the so-called Farnsworth-Hirsch fusorFusorThe Farnsworth–Hirsch fusor, or simply fusor, is an apparatus designed by Philo T. Farnsworth to create nuclear fusion. It has also been developed in various incarnations by researchers including Elmore, Tuck, and Watson, and more recently by George H. Miley and Robert W. Bussard...
) produces controlled nuclear fusionNuclear fusionNuclear fusion is the process by which two or more atomic nuclei join together, or "fuse", to form a single heavier nucleus. This is usually accompanied by the release or absorption of large quantities of energy...
by creating a dense plasma within which ionized deuteriumDeuteriumDeuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
and/or tritiumTritiumTritium is a radioactive isotope of hydrogen. The nucleus of tritium contains one proton and two neutrons, whereas the nucleus of protium contains one proton and no neutrons...
gas is heated to temperatures sufficient for creating fusion.
Light ion accelerators
- Traditional particle acceleratorParticle acceleratorA particle accelerator is a device that uses electromagnetic fields to propel charged particles to high speeds and to contain them in well-defined beams. An ordinary CRT television set is a simple form of accelerator. There are two basic types: electrostatic and oscillating field accelerators.In...
s with hydrogen (H), deuterium (D), or tritium (T) ion sources may be used to produce neutrons using targets of deuterium, tritium, lithium, beryllium, and other low-Z materials. Typically these accelerators operate with voltages in the > 1 MeV range,
High energy photoneutron/photofission
Photofission
Photofission is a process in which a nucleus, after absorbing a gamma ray, undergoes nuclear fission .Very high energy gamma rays have been shown to induce fission in elements as light as tin.-Photodisintegration:...
systems
- Neutrons (so-called photoneutrons) are produced when photons above the nuclear binding energy of a substance are incident on that substance, causing it to undergo giant dipole resonance after which it either emits a neutron (photodisintegrationPhotodisintegrationPhotodisintegration is a physical process in which an extremely high energy gamma ray interacts with an atomic nucleus and causes it to enter an excited state, which immediately decays by emitting a subatomic particle. A single proton or neutron is effectively knocked out of the nucleus by the...
) or undergoes fissionNuclear fissionIn nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
(photofissionPhotofissionPhotofission is a process in which a nucleus, after absorbing a gamma ray, undergoes nuclear fission .Very high energy gamma rays have been shown to induce fission in elements as light as tin.-Photodisintegration:...
). The number of neutrons released by each fission event is dependent on the substance. Typically photons begin to produce neutrons on interaction with normal matter at energies of about 7 to 40 MeVMEVMeV and meV are multiples and submultiples of the electron volt unit referring to 1,000,000 eV and 0.001 eV, respectively.Mev or MEV may refer to:In entertainment:* Musica Elettronica Viva, an Italian musical group...
, which means that megavoltage photon radiotherapy facilities may produce neutron radiationNeutron radiationNeutron radiation is a kind of ionizing radiation which consists of free neutrons. A result of nuclear fission or nuclear fusion, it consists of the release of free neutrons from atoms, and these free neutrons react with nuclei of other atoms to form new isotopes, which, in turn, may produce...
as well, and require special shielding for it. In addition, electrons of energy over about 50 MeVMEVMeV and meV are multiples and submultiples of the electron volt unit referring to 1,000,000 eV and 0.001 eV, respectively.Mev or MEV may refer to:In entertainment:* Musica Elettronica Viva, an Italian musical group...
may induce giant dipole resonance in nuclides by a mechanism which is the inverse of internal conversionInternal conversionInternal conversion is a radioactive decay process where an excited nucleus interacts with an electron in one of the lower atomic orbitals, causing the electron to be emitted from the atom. Thus, in an internal conversion process, a high-energy electron is emitted from the radioactive atom, but...
, and thus produce neutrons by a mechanism similar to that of photoneutrons.
Large devices

Nuclear fission reactors
- Nuclear fissionNuclear fissionIn nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
which takes place within in a nuclear reactorNuclear reactorA nuclear reactor is a device to initiate and control a sustained nuclear chain reaction. Most commonly they are used for generating electricity and for the propulsion of ships. Usually heat from nuclear fission is passed to a working fluid , which runs through turbines that power either ship's...
produces very large quantities of neutrons. In nuclear powerNuclear powerNuclear power is the use of sustained nuclear fission to generate heat and electricity. Nuclear power plants provide about 6% of the world's energy and 13–14% of the world's electricity, with the U.S., France, and Japan together accounting for about 50% of nuclear generated electricity...
reactors, the neutrons are no more than an unavoidable byproduct. In contrast, research reactorResearch reactorResearch reactors are nuclear reactors that serve primarily as a neutron source. They are also called non-power reactors, in contrast to power reactors that are used for electricity production, heat generation, or maritime propulsion.-Purpose:...
s are primarily operated to produce neutron beams. The Institut Laue-LangevinInstitut Laue-LangevinThe Institut Laue–Langevin, or ILL, is an internationally-financed scientific facility, situated in Grenoble, France. It is one of the world centres for research using neutrons...
source in GrenobleGrenobleGrenoble is a city in southeastern France, at the foot of the French Alps where the river Drac joins the Isère. Located in the Rhône-Alpes region, Grenoble is the capital of the department of Isère...
in France is the major fission source in Europe, the High Flux Isotope ReactorHigh Flux Isotope ReactorThe High Flux Isotope Reactor is a nuclear research reactor located at Oak Ridge National Laboratory in Oak Ridge, Tennessee, United States...
at ORNL is the equivalent in the US. Reactor neutron sources tend to require highly-enriched uranium fuel, making their construction somewhat controversial.
Spallation at particle accelerators
- A spallation source is a high-flux source in which protonProtonThe proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s that have been accelerated to high energies hit a target material, prompting the emission of neutrons. Examples are the Swiss neutron source SINQ, the British ISIS neutron sourceISIS neutron sourceISIS is a pulsed neutron and muon source. It is situated at the Rutherford Appleton Laboratory on the Harwell Science and Innovation Campus in Oxfordshire, United Kingdom and is part of the Science and Technology Facilities Council...
, and the U.S. Spallation Neutron SourceSpallation Neutron SourceThe Spallation Neutron Source is an accelerator-based neutron source facility that provides the most intense pulsed neutron beams in the world for scientific research and industrial development...
; the China Spallation Neutron SourceChina spallation neutron sourceThe China Spallation Neutron Source is a proposed accelerator-based neutron source, operated by the Institute of High Energy Physics, to be built at Dongguan in Guangdong province - the first major scientific facility in south China...
is under construction in DongguanDongguanDongguan is a prefecture-level city in central Guangdong province, People's Republic of China.An important industrial city located in the Pearl River Delta, Dongguan borders the provincial capital of Guangzhou to the north, Huizhou to the northeast, Shenzhen to the south, and the Pearl River to...
Nuclear fusion systems
- Nuclear fusionNuclear fusionNuclear fusion is the process by which two or more atomic nuclei join together, or "fuse", to form a single heavier nucleus. This is usually accompanied by the release or absorption of large quantities of energy...
, the combining of the heavy isotopes of hydrogen, also has the potential to produce large quantities of neutrons. Small scale fusion systems exist for research purposes at many universities and laboratories around the world. A small number of large scale nuclear fusion systems also exist including the National Ignition FacilityNational Ignition FacilityThe National Ignition Facility, or NIF is a large, laser-based inertial confinement fusion research device located at the Lawrence Livermore National Laboratory in Livermore, California. NIF uses powerful lasers to heat and compress a small amount of hydrogen fuel to the point where nuclear fusion...
in the USA, JETJoint European TorusJET, the Joint European Torus, is the largest magnetic confinement plasma physics experiment worldwide currently in operation. Its main purpose is to open the way to future nuclear fusion experimental tokamak reactors such as ITER and :DEMO....
in the UK, and soon the recently started ITERITERITER is an international nuclear fusion research and engineering project, which is currently building the world's largest and most advanced experimental tokamak nuclear fusion reactor at Cadarache in the south of France...
experiment in France.
Neutron flux density
For most applications, a higher neutron fluxNeutron flux
The neutron flux is a quantity used in reactor physics corresponding to the total length travelled by all neutrons per unit time and volume . The neutron fluence is defined as the neutron flux integrated over a certain time period....
is always better (since it reduces the time required to conduct the experiment, acquire the image, etc.). Amateur fusion devices, like the fusor
Fusor
The Farnsworth–Hirsch fusor, or simply fusor, is an apparatus designed by Philo T. Farnsworth to create nuclear fusion. It has also been developed in various incarnations by researchers including Elmore, Tuck, and Watson, and more recently by George H. Miley and Robert W. Bussard...
, generate only about 300 000 neutrons per second. Commercial fusor devices can generate on the order of 109 neutrons per second, which corresponds to a usable flux of less than 105 n/(cm² s). Large neutron beamlines around the world achieve much greater flux. Reactor-based sources now produce 1015 n/(cm² s), and spallation sources generate greater than 1017 n/(cm² s).
See also
- Astronomical neutron source
- Nuclear fissionNuclear fissionIn nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
- Neutron generatorNeutron generatorNeutron generators are neutron source devices which contain compact linear accelerators and that produce neutrons by fusing isotopes of hydrogen together. The fusion reactions take place in these devices by accelerating either deuterium, tritium, or a mixture of these two isotopes into a metal...
- Radioactive decayRadioactive decayRadioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
- Radioactivity

