
Mercury battery
Encyclopedia
A mercury battery is a non-rechargeable electrochemical battery
, a primary cell
. Due to the content of mercury
, and the resulting environmental concerns, the sale of mercury batteries is banned in many countries. Both ANSI
and IEC
have withdrawn standards for mercury batteries. Mercury batteries were made in button types for watches, hearing aids, and calculators, and in larger forms for other applications.
developed a balanced mercury cell which was useful for military applications such as metal detectors, munitions, and walkie-talkie
s. The battery system had the advantages of long shelf life (to 10 years) and steady voltage output. After the Second World War the battery system was widely applied for small electronic devices such as cardiac pacemaker
s and hearing aid
s. Mercury oxide batteries were made in a range of sizes from miniature button cell
s used for hearing aid
s and electric wrist watches, cylindrical types used for portable electronic apparatus, rectangular batteries used for transistor radios, and large multicell packs used for industrial applications such as radio remote control
for overhead crane systems. In the United States, mercury oxide batteries were manufactured by companies including P. R. Mallory and Co Inc
, (now Duracell
), Union Carbide Corporation (whose former battery division is now called Energizer Holdings
), RCA Corporation, and Burgess Battery Company.
or a mixture of mercuric oxide with manganese dioxide as the cathode
. Mercuric oxide is a non-conductor so some graphite is mixed with it; the graphite also helps prevent collection of mercury into large droplets. The anode
is made of zinc
and separated from the cathode with a layer of paper or other porous material soaked with electrolyte. During discharge, zinc oxidizes to zinc oxide
and mercuric oxide gets reduced to elemental mercury. A little extra mercuric oxide is put into the cell to prevent evolution of hydrogen gas at the end of life. Mercury batteries are very similar to silver-oxide batteries
.
The overall reaction is:
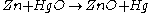
Sodium hydroxide or potassium hydroxide
are used as an electrolyte
. Sodium hydroxide cells have nearly constant voltage at low discharge currents, making them ideal for hearing aids, calculator
s, and electronic watch
es. Potassium hydroxide cells, in turn, provided constant voltage at higher currents, making them suitable for applications requiring current surges, e.g. photographic cameras with flash, and watches with a backlight. Potassium hydroxide cells also have better performance at lower temperatures. Mercury cells have very long shelf life, up to 10 years.
A different form of mercury battery uses mercuric oxide and cadmium
. This has a much lower terminal voltage around 0.9 volts and so has lower energy density, but it has an extended temperature range, in special designs up to 180 C. A 12 volt battery of this type was formerly used for residential smoke detector
s, where the two-step voltage characteristic gave a useful warning for replacement.
cathode
have a very flat discharge curve, holding constant 1.35 V (open circuit) voltage until about the last 5% of their lifetime, when their voltage drops rapidly. The voltage remains within 1% for several years at light load, and over a wide temperature range, making mercury batteries useful as a reference voltage in electronic instruments and in photographic light meter
s. Mercury batteries with cathodes made of a mix of mercuric oxide and manganese dioxide have output voltage of 1.4 V and more sloped discharge curve.
directive 91/157
, when adopted by member states, prohibited the marketing of certain types of batteries containing more than 25 milligrams of mercury, or, in the case of alkaline batteries
, more than 0.025 % by weight of mercury. In 1998 the ban was extended to cells containing more than 0.005% by weight of mercury.
In the United States, in 1992 the state of New Jersey
prohibited sales of mercury batteries. In 1996 the United States Congress passed the Mercury-Containing and Rechargeable Battery Management Act
that prohibited further sale of mercury-containing batteries unless manufacturers provided a reclamation facility, effectively banning the sale.
, with similar discharge curve, high capacity, but much shorter lifetime (a few months) and poor performance in dry climates, alkaline batteries
with voltage widely varying through their lifetime, and silver-oxide batteries
with higher voltage (1.55 V) and very flat discharge curve, making them possibly the best, though expensive, replacement after recalibrating the meter to the new voltage. Special adapters with voltage dropping Schottky
or Germanium diodes are available, to adapt silver oxide batteries for use in older equipment designed for mercury batteries, such as cameras and light meters which require a stable, exact voltage, however, since the voltage drop is a non-linear function of the current flow, diodes don't represent a very accurate solution for applications where the current flow alters significantly. Currents drawn by old CdS light meters are typically in the 10 µA to 200 µA range (Minolta SR-T range). Various kinds of active voltage regulation circuits using SMD transistor based or integrated ultra-low-drop ultra-low-power 1.35 V voltage regulators or bandgap voltage references have been devised, however, they are often difficult to integrate into the (battery compartment of a) camera because of space constraints or the fact that many old cameras are hard-wired to use the chassis for the battery's positive pole, requiring the adoption of a less-common low-side regulator design. Other things making such circuits difficult to design are the very low supply voltage of typically just 1.4 V to 1.6 V (a single battery cell) with a maximum allowed voltage drop in the 50 mV range and the fact that with dark-currents (with lens cap on) in the 10 µA range, many old light meters don't even provide a power switch, making an ultra-low-power design for any kind of replacement circuitry mandantory.
Battery (electricity)
An electrical battery is one or more electrochemical cells that convert stored chemical energy into electrical energy. Since the invention of the first battery in 1800 by Alessandro Volta and especially since the technically improved Daniell cell in 1836, batteries have become a common power...
, a primary cell
Primary cell
A primary cell is any kind of battery in which the electrochemical reaction is not reversible, rendering the cell non-rechargeable. A common example of a primary cell is the disposable battery. Unlike a secondary cell, the reaction cannot be reversed by running a current into the cell; the chemical...
. Due to the content of mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
, and the resulting environmental concerns, the sale of mercury batteries is banned in many countries. Both ANSI
Ansi
Ansi is a village in Kaarma Parish, Saare County, on the island of Saaremaa, Estonia....
and IEC
International Electrotechnical Commission
The International Electrotechnical Commission is a non-profit, non-governmental international standards organization that prepares and publishes International Standards for all electrical, electronic and related technologies – collectively known as "electrotechnology"...
have withdrawn standards for mercury batteries. Mercury batteries were made in button types for watches, hearing aids, and calculators, and in larger forms for other applications.
History
The mercury oxide-zinc battery system was known more than 100 years ago but did not become widely used until 1942, when Samuel RubenSamuel Ruben
Samuel Ruben was an inventor who made lasting contributions to electrochemistry and solid-state technology, including the founding of Duracell.-Early life:...
developed a balanced mercury cell which was useful for military applications such as metal detectors, munitions, and walkie-talkie
Walkie-talkie
A walkie-talkie is a hand-held, portable, two-way radio transceiver. Its development during the Second World War has been variously credited to Donald L. Hings, radio engineer Alfred J. Gross, and engineering teams at Motorola...
s. The battery system had the advantages of long shelf life (to 10 years) and steady voltage output. After the Second World War the battery system was widely applied for small electronic devices such as cardiac pacemaker
Pacemaker
An artificial pacemaker is a medical device that uses electrical impulses to regulate the beating of the heart.Pacemaker may also refer to:-Medicine:...
s and hearing aid
Hearing aid
A hearing aid is an electroacoustic device which typically fits in or behind the wearer's ear, and is designed to amplify and modulate sound for the wearer. Earlier devices, known as "ear trumpets" or "ear horns", were passive funnel-like amplification cones designed to gather sound energy and...
s. Mercury oxide batteries were made in a range of sizes from miniature button cell
Button cell
A watch battery or button cell is a small single cell battery shaped as a squat cylinder typically 5 to 12 mm in diameter and 1 to 6 mm high—like a button on a garment, hence the name. Button cells are used to power small portable electronics devices such as wrist watches, pocket...
s used for hearing aid
Hearing aid
A hearing aid is an electroacoustic device which typically fits in or behind the wearer's ear, and is designed to amplify and modulate sound for the wearer. Earlier devices, known as "ear trumpets" or "ear horns", were passive funnel-like amplification cones designed to gather sound energy and...
s and electric wrist watches, cylindrical types used for portable electronic apparatus, rectangular batteries used for transistor radios, and large multicell packs used for industrial applications such as radio remote control
Remote control
A remote control is a component of an electronics device, most commonly a television set, used for operating the television device wirelessly from a short line-of-sight distance.The remote control is usually contracted to remote...
for overhead crane systems. In the United States, mercury oxide batteries were manufactured by companies including P. R. Mallory and Co Inc
P. R. Mallory and Co Inc
P. R. Mallory and Co Inc was a US producer of dry cell batteries , electronic components including electrolytic capacitors, and audible warning devices , it also was the parent firm of Mallory Batteries Ltd., an Irish producer of Ever Ready batteries...
, (now Duracell
Duracell
Duracell is a brand of batteries manufactured by Procter & Gamble.Additionally, Duracell owns the Procell professional-use brand.-Products:Duracell manufactures alkaline batteries in many common sizes, such as AAA, AA, C, D, and 9V...
), Union Carbide Corporation (whose former battery division is now called Energizer Holdings
Energizer Holdings
Energizer Holdings is an American manufacturer of batteries and personal care products, headquartered in Town and Country, Missouri. Its most well known brands are Energizer and Eveready batteries, Schick, Wilkinson Sword and Edge shaving products, Playtex feminine hygiene and baby products, and...
), RCA Corporation, and Burgess Battery Company.
Chemistry
Mercury batteries use either pure mercuric oxideMercury(II) oxide
Mercury oxide, also called mercuric oxide or simply mercury oxide, has a formula of HgO. It has a red or orange color. Mercury oxide is a solid at room temperature and pressure...
or a mixture of mercuric oxide with manganese dioxide as the cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
. Mercuric oxide is a non-conductor so some graphite is mixed with it; the graphite also helps prevent collection of mercury into large droplets. The anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
is made of zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
and separated from the cathode with a layer of paper or other porous material soaked with electrolyte. During discharge, zinc oxidizes to zinc oxide
Zinc oxide
Zinc oxide is an inorganic compound with the formula ZnO. It is a white powder that is insoluble in water. The powder is widely used as an additive into numerous materials and products including plastics, ceramics, glass, cement, rubber , lubricants, paints, ointments, adhesives, sealants,...
and mercuric oxide gets reduced to elemental mercury. A little extra mercuric oxide is put into the cell to prevent evolution of hydrogen gas at the end of life. Mercury batteries are very similar to silver-oxide batteries
Silver-oxide battery
A silver oxide battery , not to be confused with a similar but different silver–zinc battery, which is a secondary cell, is a primary cell with relatively very high energy/weight ratio. They are costly due to the high price of silver...
.
The overall reaction is:
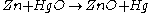
Sodium hydroxide or potassium hydroxide
Potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
are used as an electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
. Sodium hydroxide cells have nearly constant voltage at low discharge currents, making them ideal for hearing aids, calculator
Calculator
An electronic calculator is a small, portable, usually inexpensive electronic device used to perform the basic operations of arithmetic. Modern calculators are more portable than most computers, though most PDAs are comparable in size to handheld calculators.The first solid-state electronic...
s, and electronic watch
Watch
A watch is a small timepiece, typically worn either on the wrist or attached on a chain and carried in a pocket, with wristwatches being the most common type of watch used today. They evolved in the 17th century from spring powered clocks, which appeared in the 15th century. The first watches were...
es. Potassium hydroxide cells, in turn, provided constant voltage at higher currents, making them suitable for applications requiring current surges, e.g. photographic cameras with flash, and watches with a backlight. Potassium hydroxide cells also have better performance at lower temperatures. Mercury cells have very long shelf life, up to 10 years.
A different form of mercury battery uses mercuric oxide and cadmium
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
. This has a much lower terminal voltage around 0.9 volts and so has lower energy density, but it has an extended temperature range, in special designs up to 180 C. A 12 volt battery of this type was formerly used for residential smoke detector
Smoke detector
A smoke detector is a device that detects smoke, typically as an indicator of fire. Commercial, industrial, and mass residential devices issue a signal to a fire alarm system, while household detectors, known as smoke alarms, generally issue a local audible and/or visual alarm from the detector...
s, where the two-step voltage characteristic gave a useful warning for replacement.
Electrical characteristics
Mercury batteries using mercury(II) oxideMercury(II) oxide
Mercury oxide, also called mercuric oxide or simply mercury oxide, has a formula of HgO. It has a red or orange color. Mercury oxide is a solid at room temperature and pressure...
cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
have a very flat discharge curve, holding constant 1.35 V (open circuit) voltage until about the last 5% of their lifetime, when their voltage drops rapidly. The voltage remains within 1% for several years at light load, and over a wide temperature range, making mercury batteries useful as a reference voltage in electronic instruments and in photographic light meter
Light meter
A light meter is a device used to measure the amount of light. In photography, a light meter is often used to determine the proper exposure for a photograph...
s. Mercury batteries with cathodes made of a mix of mercuric oxide and manganese dioxide have output voltage of 1.4 V and more sloped discharge curve.
Product ban
The 1991 European commissionEuropean Commission
The European Commission is the executive body of the European Union. The body is responsible for proposing legislation, implementing decisions, upholding the Union's treaties and the general day-to-day running of the Union....
directive 91/157
Battery Directive
Directive 2006/66/EC of the European Parliament and of the Council of 6 September 2006 on batteries and accumulators and waste batteries and accumulators and repealing Directive 91/157/EEC, commonly known as the Battery Directive, regulates the manufacture and disposal of batteries in the European...
, when adopted by member states, prohibited the marketing of certain types of batteries containing more than 25 milligrams of mercury, or, in the case of alkaline batteries
Alkaline battery
Alkaline batteries are a type of primary batteries dependent upon the reaction between zinc and manganese dioxide . A rechargeable alkaline battery allows reuse of specially designed cells....
, more than 0.025 % by weight of mercury. In 1998 the ban was extended to cells containing more than 0.005% by weight of mercury.
In the United States, in 1992 the state of New Jersey
New Jersey
New Jersey is a state in the Northeastern and Middle Atlantic regions of the United States. , its population was 8,791,894. It is bordered on the north and east by the state of New York, on the southeast and south by the Atlantic Ocean, on the west by Pennsylvania and on the southwest by Delaware...
prohibited sales of mercury batteries. In 1996 the United States Congress passed the Mercury-Containing and Rechargeable Battery Management Act
Mercury-containing and Rechargeable Battery Management Act
In the United States, the Mercury-Containing and Rechargeable Battery Management Act was signed into law on May 13, 1996...
that prohibited further sale of mercury-containing batteries unless manufacturers provided a reclamation facility, effectively banning the sale.
Substitutes
The ban on sale of mercury oxide batteries caused numerous problems for photographers, whose equipment frequently relied on their advantageous discharge curves and long lifetime. Alternatives used are zinc-air batteriesZinc-air battery
Zinc–air batteries , and zinc–air fuel cells, are electro-chemical batteries powered by oxidizing zinc with oxygen from the air. These batteries have high energy densities and are relatively inexpensive to produce...
, with similar discharge curve, high capacity, but much shorter lifetime (a few months) and poor performance in dry climates, alkaline batteries
Alkaline battery
Alkaline batteries are a type of primary batteries dependent upon the reaction between zinc and manganese dioxide . A rechargeable alkaline battery allows reuse of specially designed cells....
with voltage widely varying through their lifetime, and silver-oxide batteries
Silver-oxide battery
A silver oxide battery , not to be confused with a similar but different silver–zinc battery, which is a secondary cell, is a primary cell with relatively very high energy/weight ratio. They are costly due to the high price of silver...
with higher voltage (1.55 V) and very flat discharge curve, making them possibly the best, though expensive, replacement after recalibrating the meter to the new voltage. Special adapters with voltage dropping Schottky
Schottky diode
The Schottky diode is a semiconductor diode with a low forward voltage drop and a very fast switching action...
or Germanium diodes are available, to adapt silver oxide batteries for use in older equipment designed for mercury batteries, such as cameras and light meters which require a stable, exact voltage, however, since the voltage drop is a non-linear function of the current flow, diodes don't represent a very accurate solution for applications where the current flow alters significantly. Currents drawn by old CdS light meters are typically in the 10 µA to 200 µA range (Minolta SR-T range). Various kinds of active voltage regulation circuits using SMD transistor based or integrated ultra-low-drop ultra-low-power 1.35 V voltage regulators or bandgap voltage references have been devised, however, they are often difficult to integrate into the (battery compartment of a) camera because of space constraints or the fact that many old cameras are hard-wired to use the chassis for the battery's positive pole, requiring the adoption of a less-common low-side regulator design. Other things making such circuits difficult to design are the very low supply voltage of typically just 1.4 V to 1.6 V (a single battery cell) with a maximum allowed voltage drop in the 50 mV range and the fact that with dark-currents (with lens cap on) in the 10 µA range, many old light meters don't even provide a power switch, making an ultra-low-power design for any kind of replacement circuitry mandantory.

