Longifolene
Encyclopedia
Longifolene is the common (or trivial) chemical name of a naturally occurring, oily liquid hydrocarbon
found primarily in the high-boiling fraction of certain pine resin
s. The name is derived from that of a pine
species from which the compound was isolated, Pinus longifolia (obsolete name for Pinus roxburghii Sarg.)
Chemically, longifolene is a tricyclic sesquiterpene
. This molecule is chiral
, and the enantiomer
commonly found in pines and other higher plants exhibits a positive optical rotation
of +42.73°. The other enantiomer (optical rotation −42.73°) is found in small amounts in certain fungi and liverworts.
Longifolene is used in organic synthesis for the preparation of dilongifolylborane, a chiral hydroborating
agent.
Longifolene is also one of two most abundant aroma constituents of lapsang souchong
tea, because the tea is smoked over pine fires.
s, Longifolene is an attractive target for research groups highlighting new synthetic methodologies. Notable syntheses are by Corey
, McMurray, Johnson, Oppolzer, and Schultz.
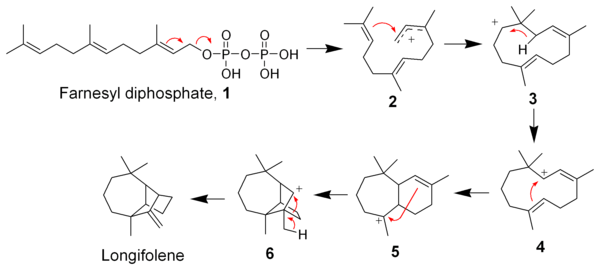
The Johnson biosynthesis has since been validated as feasible using modern quantum mechanical computational methods. The subsequent cationic cascade mechanism has been shown to go through a non-classical cation intermediate.
) by means of a cationic polycyclization cascade. Loss of the pyrophosphate group and cyclization by the distal alkene
gives intermediate 3, which by means of a 1,3-hydride shift gives intermediate 4. After two additional cyclizations, intermediate 6 produces longifolene by a 1,2-alkyl migration
.
as a chiral hydroborating agent.
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
found primarily in the high-boiling fraction of certain pine resin
Resin
Resin in the most specific use of the term is a hydrocarbon secretion of many plants, particularly coniferous trees. Resins are valued for their chemical properties and associated uses, such as the production of varnishes, adhesives, and food glazing agents; as an important source of raw materials...
s. The name is derived from that of a pine
Pine
Pines are trees in the genus Pinus ,in the family Pinaceae. They make up the monotypic subfamily Pinoideae. There are about 115 species of pine, although different authorities accept between 105 and 125 species.-Etymology:...
species from which the compound was isolated, Pinus longifolia (obsolete name for Pinus roxburghii Sarg.)
Chemically, longifolene is a tricyclic sesquiterpene
Terpene
Terpenes are a large and diverse class of organic compounds, produced by a variety of plants, particularly conifers, though also by some insects such as termites or swallowtail butterflies, which emit terpenes from their osmeterium. They are often strong smelling and thus may have had a protective...
. This molecule is chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
, and the enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
commonly found in pines and other higher plants exhibits a positive optical rotation
Optical rotation
Optical rotation is the turning of the plane of linearly polarized light about the direction of motion as the light travels through certain materials. It occurs in solutions of chiral molecules such as sucrose , solids with rotated crystal planes such as quartz, and spin-polarized gases of atoms...
of +42.73°. The other enantiomer (optical rotation −42.73°) is found in small amounts in certain fungi and liverworts.
Longifolene is used in organic synthesis for the preparation of dilongifolylborane, a chiral hydroborating
Hydroboration-oxidation reaction
In organic chemistry, the hydroboration–oxidation reaction is a two-step organic reaction that converts an alkene into a neutral alcohol by the net addition of water across the double bond. The hydrogen and hydroxyl group are added in a syn addition leading to cis stereochemistry...
agent.
Longifolene is also one of two most abundant aroma constituents of lapsang souchong
Lapsang souchong
Lapsang souchong is a black tea originally from the Wuyi region of the Chinese province of Fujian. It is sometimes referred to as smoked tea...
tea, because the tea is smoked over pine fires.
Total syntheses
Due to the compact tricyclic structure and lack of functional groupFunctional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s, Longifolene is an attractive target for research groups highlighting new synthetic methodologies. Notable syntheses are by Corey
Elias James Corey
Elias James Corey is an American organic chemist. In 1990 he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis...
, McMurray, Johnson, Oppolzer, and Schultz.
| Longifolene total synthesis by Corey.svg |
|---|
The Johnson biosynthesis has since been validated as feasible using modern quantum mechanical computational methods. The subsequent cationic cascade mechanism has been shown to go through a non-classical cation intermediate.
Biosynthesis
The biosynthesis of longifolene begins with farnesyl diphosphate (1) (also called farnesyl pyrophosphateFarnesyl pyrophosphate
Farnesyl pyrophosphate is an intermediate in the HMG-CoA reductase pathway used by organisms in the biosynthesis of terpenes, terpenoids, and sterols...
) by means of a cationic polycyclization cascade. Loss of the pyrophosphate group and cyclization by the distal alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
gives intermediate 3, which by means of a 1,3-hydride shift gives intermediate 4. After two additional cyclizations, intermediate 6 produces longifolene by a 1,2-alkyl migration
1,2-rearrangement
A 1,2-rearrangement or 1,2-migration or 1,2-shift or Whitmore 1,2-shift is an organic reaction where a substituent moves from one atom to another atom in a chemical compound. In a 1,2 shift the movement involves two adjacent atoms but moves over larger distances are possible...
.

Use
The borane derivative dilongifolylborane is used in organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
as a chiral hydroborating agent.

