
Lattice energy
Encyclopedia
Ionic bond
An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. Pure ionic bonding cannot exist: all ionic compounds have some...
solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
is a measure of the strength of bonds in that ionic compound. It is usually defined as the enthalpy of formation
Standard enthalpy change of formation
The standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy that accompanies the formation of 1 mole of a substance in its standard state from its constituent elements in their standard states...
of the ionic compound
Ionic compound
In chemistry, an ionic compound is a chemical compound in which ions are held together in a lattice structure by ionic bonds. Usually, the positively charged portion consists of metal cations and the negatively charged portion is an anion or polyatomic ion. Ions in ionic compounds are held together...
from gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
eous ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s and as such is invariably exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
. Lattice energy may also be defined as the energy required to completely separate one mole of a solid ionic compound into gaseous ionic constituents. The concept of lattice energy was initially developed for rocksalt-structured and sphalerite
Sphalerite
Sphalerite is a mineral that is the chief ore of zinc. It consists largely of zinc sulfide in crystalline form but almost always contains variable iron. When iron content is high it is an opaque black variety, marmatite. It is usually found in association with galena, pyrite, and other sulfides...
-structured compounds like NaCl and ZnS, where the ions occupy high-symmetry crystal lattice sites. In the case of NaCl, the lattice energy is the energy released by the reaction
- Na+ (g) + Cl− (g) → NaCl (s)
which would amount to -787 kJ/mol.
Some older textbooks define lattice energy as the energy required to convert the ionic compound into gaseous ions which is an endothermic
Endothermic
In thermodynamics, the word endothermic describes a process or reaction in which the system absorbs energy from the surroundings in the form of heat. Its etymology stems from the prefix endo- and the Greek word thermasi,...
process, and following this definition the lattice energy of NaCl would be +787 kJ/mol.
The precise value of the lattice energy may not be determined experimentally, because of the impossibility of preparing an adequate amount of gaseous anions and cations and measuring the energy released during their condensation to form the solid. However, the value of the lattice energy may either be derived theoretically from electrostatics
Electrostatics
Electrostatics is the branch of physics that deals with the phenomena and properties of stationary or slow-moving electric charges....
or from a thermodynamic cycling reaction, the so-called Born-Haber cycle
Born-Haber cycle
The Born–Haber cycle is an approach to analyzing reaction energies. It was named after and developed by the two German scientists Max Born and Fritz Haber....
.
Born-Landé equation
In 1918 BornMax Born
Max Born was a German-born physicist and mathematician who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics and supervised the work of a number of notable physicists in the 1920s and 30s...
and Landé
Alfred Landé
Alfred Landé was a German-American physicist known for his contributions to quantum theory. He is responsible for the Landé g-factor an explanation of the Zeeman Effect.-Life and Achievements:...
proposed that the lattice energy could be derived from the electric potential
Electric potential
In classical electromagnetism, the electric potential at a point within a defined space is equal to the electric potential energy at that location divided by the charge there...
of the ionic lattice and a repulsive potential energy
Potential energy
In physics, potential energy is the energy stored in a body or in a system due to its position in a force field or due to its configuration. The SI unit of measure for energy and work is the Joule...
term.
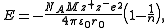
where
- NA is the Avogadro constant;
- M is the Madelung constantMadelung constantThe Madelung constant is used in determining the electrostatic potential of a single ion in a crystal by approximating the ions by point charges. It is named after Erwin Madelung, a German physicist....
, relating to the geometry of the crystal; - z+ is the charge number of cation;
- z− is the charge number of anion;
- e is the elementary chargeElementary chargeThe elementary charge, usually denoted as e, is the electric charge carried by a single proton, or equivalently, the absolute value of the electric charge carried by a single electron. This elementary charge is a fundamental physical constant. To avoid confusion over its sign, e is sometimes called...
, equal to ; - ε0 is the permittivity of free space, equal to ;
- r0 is the distance to closest ion; and
- n is the Born exponent, a number between 5 and 12, determined experimentally by measuring the compressibility of the solid, or derived theoretically.
The Born-Landé equation gives a reasonable fit to the lattice energy.
| Compound | Calculated Lattice Energy | Experimental Lattice Energy |
|---|---|---|
| NaCl | −756 kJ/mol | −787 kJ/mol |
| LiF | −1007 kJ/mol | −1046 kJ/mol |
| CaCl2 | −2170 kJ/mol | −2255 kJ/mol |
From the Born-Landé equation it can be seen that the lattice energy of a compound is dependent on a number of factors
- as the charges on the ions increase the lattice energy increases (becomes more negative),
- when ions are closer together the lattice energy increases (becomes more negative)
Barium oxide (BaO), for instance, which has the NaCl structure and therefore the same Madelung constant, has a bond radius of 275 picometers and a lattice energy of -3054 kJ/mol, while sodium chloride (NaCl) has a bond radius of 283 picometers and a lattice energy of -786 kJ/mol.
Kapustinskii equation
The Kapustinskii equationKapustinskii equation
The Kapustinskii equation calculates the Lattice Energy UL for an ionic crystal, which is experimentally difficult to determine. It is named after Anatoli Fedorovich Kapustinskii who published the formula in 1956....
can be used as a simpler way of deriving lattice energies where high precision is not required.
Effect of polarisation
For ionic compounds with ions occupying lattice sites with crystallographic point groups C1, C1h, Cn or Cnv (n = 2, 3, 4 or 6) the concept of the lattice energy and the Born-Haber cycle has to be extended. In these cases the polarization energy Epol associated with ions on polar lattice sites has to be included in the Born-Haber cycle and the solid formation reaction has to start from the already polarized species. As an example, one may consider the case of iron-pyritePyrite
The mineral pyrite, or iron pyrite, is an iron sulfide with the formula FeS2. This mineral's metallic luster and pale-to-normal, brass-yellow hue have earned it the nickname fool's gold because of its resemblance to gold...
FeS2, where sulfur ions occupy lattice site of point symmetry group C3. The lattice energy defining reaction then reads
- Fe2+ (g) + 2 pol S− (g) → FeS2 (s)
where pol S− stands for the polarized, gaseous sulfur ion. It has been shown that the neglection of the effect led to 15 % difference between theoretical and experimental thermodynamic cycle energy of FeS2 that reduced to only 2%, when the sulfur polarization effects were included.

