Born-Haber cycle
Encyclopedia
The Born–Haber cycle is an approach to analyzing reaction energies
. It was named after and developed by the two German
scientists Max Born
and Fritz Haber
.
The Born–Haber cycle involves the formation of an ionic compound
from the reaction of a metal
(often a Group I
or Group II
element) with a non-metal. Born–Haber cycles are used primarily as a means of calculating lattice energies (or more precisely enthalpies) which cannot otherwise be measured directly.
The lattice enthalpy is the enthalpy
change involved in formation of the ionic compound from gaseous ions. Some chemists define it as the energy to break the ionic compound into gaseous ions. The former definition is invariably exothermic
and the latter is endothermic
.
A Born–Haber cycle calculates the lattice enthalpy by comparing the standard enthalpy change of formation
of the ionic compound (from the elements) to the enthalpy required to make gaseous ions from the elements
. This is an application of Hess's Law
.
This latter calculation is complex. To make gaseous ions from elements it is necessary to atomise the elements (turn each into gaseous atoms) and then to ionise the atoms. If the element is normally a molecule then we have to consider its bond dissociation enthalpy (see also bond energy
). The energy required to remove one or more electron
s to make a cation is a sum of successive ionization energies
; for example the energy needed to form Mg2+ is the first plus the second ionization energies of Mg. The energy released when one electron is added to an atom to make it an anion is called the electron affinity
.
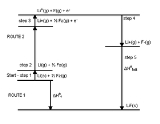
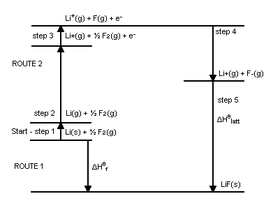 The enthalpy of formation of lithium fluoride
The enthalpy of formation of lithium fluoride
from its elements lithium and fluorine in their stable forms is modeled in five steps in the diagram:
The same calculation applies for any metal other than lithium or any non-metal other than fluorine.
The sum of the energies for each step of the process must equal the enthalpy of formation of the metal and non-metal,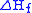 .
.

The net enthalpy of formation and the first four of the five energies can be determined experimentally, but the lattice energy cannot be measured directly. Instead, the lattice energy is calculated by subtracting the other four energies in the Born–Haber cycle from the net enthalpy of formation.
The word cycle refers to the fact that one can also equate to zero the total enthalpy change for a cyclic process, starting and ending with LiF(s) in the example. This leads to
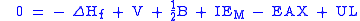
which is equivalent to the previous equation.
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
. It was named after and developed by the two German
Germans
The Germans are a Germanic ethnic group native to Central Europe. The English term Germans has referred to the German-speaking population of the Holy Roman Empire since the Late Middle Ages....
scientists Max Born
Max Born
Max Born was a German-born physicist and mathematician who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics and supervised the work of a number of notable physicists in the 1920s and 30s...
and Fritz Haber
Fritz Haber
Fritz Haber was a German chemist, who received the Nobel Prize in Chemistry in 1918 for his development for synthesizing ammonia, important for fertilizers and explosives. Haber, along with Max Born, proposed the Born–Haber cycle as a method for evaluating the lattice energy of an ionic solid...
.
The Born–Haber cycle involves the formation of an ionic compound
Ionic compound
In chemistry, an ionic compound is a chemical compound in which ions are held together in a lattice structure by ionic bonds. Usually, the positively charged portion consists of metal cations and the negatively charged portion is an anion or polyatomic ion. Ions in ionic compounds are held together...
from the reaction of a metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
(often a Group I
Alkali metal
The alkali metals are a series of chemical elements in the periodic table. In the modern IUPAC nomenclature, the alkali metals comprise the group 1 elements, along with hydrogen. The alkali metals are lithium , sodium , potassium , rubidium , caesium , and francium...
or Group II
Alkaline earth metal
The alkaline earth metals are a group in the periodic table. In the modern IUPAC nomenclature, the alkaline earth metals are called the group 2 elements. Previously, they were called the Group IIA elements . The alkaline earth metals contain beryllium , magnesium , calcium , strontium , barium and...
element) with a non-metal. Born–Haber cycles are used primarily as a means of calculating lattice energies (or more precisely enthalpies) which cannot otherwise be measured directly.
The lattice enthalpy is the enthalpy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
change involved in formation of the ionic compound from gaseous ions. Some chemists define it as the energy to break the ionic compound into gaseous ions. The former definition is invariably exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
and the latter is endothermic
Endothermic
In thermodynamics, the word endothermic describes a process or reaction in which the system absorbs energy from the surroundings in the form of heat. Its etymology stems from the prefix endo- and the Greek word thermasi,...
.
A Born–Haber cycle calculates the lattice enthalpy by comparing the standard enthalpy change of formation
Standard enthalpy change of formation
The standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy that accompanies the formation of 1 mole of a substance in its standard state from its constituent elements in their standard states...
of the ionic compound (from the elements) to the enthalpy required to make gaseous ions from the elements
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
. This is an application of Hess's Law
Hess's law
Hess' law is a relationship in physical chemistry named for Germain Hess, a Swiss-born Russian chemist and physician.The law states that the enthalpy change for a reaction that is carried out in a series of steps is equal to the sum of the enthalpy changes for the individual steps.The law is an...
.
This latter calculation is complex. To make gaseous ions from elements it is necessary to atomise the elements (turn each into gaseous atoms) and then to ionise the atoms. If the element is normally a molecule then we have to consider its bond dissociation enthalpy (see also bond energy
Bond energy
In chemistry, bond energy is the measure of bond strength in a chemical bond. It is the heat required to break one Mole of molecules into their individual atoms. For example, the carbon-hydrogen bond energy in methane E is the enthalpy change involved with breaking up one molecule of methane into...
). The energy required to remove one or more electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s to make a cation is a sum of successive ionization energies
Ionization energy
The ionization energy of a chemical species, i.e. an atom or molecule, is the energy required to remove an electron from the species to a practically infinite distance. Large atoms or molecules have a low ionization energy, while small molecules tend to have higher ionization energies.The property...
; for example the energy needed to form Mg2+ is the first plus the second ionization energies of Mg. The energy released when one electron is added to an atom to make it an anion is called the electron affinity
Electron affinity
The Electron affinity of an atom or molecule is defined as the amount of energy released when an electron is added to a neutral atom or molecule to form a negative ion....
.
Example: formation of lithium fluoride

Lithium fluoride
Lithium fluoride is an inorganic compound with the formula LiF. It is the lithium salt of hydrofluoric acid. This white solid is a simple ionic compound. Its structure is analogous to that of sodium chloride, but it is much less soluble in water. It is mainly used as a component of molten...
from its elements lithium and fluorine in their stable forms is modeled in five steps in the diagram:
- Atomisation enthalpy of lithium
- Ionization enthalpy of lithium
- Atomisation enthalpy of fluorine
- Electron affinity of fluorine
- Lattice enthalpy
The same calculation applies for any metal other than lithium or any non-metal other than fluorine.
The sum of the energies for each step of the process must equal the enthalpy of formation of the metal and non-metal,
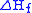 .
.
- V is the enthalpy of sublimationEnthalpy of sublimationThe enthalpy of sublimation, or heat of sublimation, is defined as the heat required to sublime one mole of the substance at a given combination of temperature and pressure, usually standard temperature and pressure...
for metal atoms (lithium) - B is the bond energy (of F2). The coefficient 1/2 is used because the formation reaction is Li + 1/2 F2 → LiF.
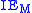 is the ionization energyIonization energyThe ionization energy of a chemical species, i.e. an atom or molecule, is the energy required to remove an electron from the species to a practically infinite distance. Large atoms or molecules have a low ionization energy, while small molecules tend to have higher ionization energies.The property...
is the ionization energyIonization energyThe ionization energy of a chemical species, i.e. an atom or molecule, is the energy required to remove an electron from the species to a practically infinite distance. Large atoms or molecules have a low ionization energy, while small molecules tend to have higher ionization energies.The property...
of the metal atom:
 is the electron affinityElectron affinityThe Electron affinity of an atom or molecule is defined as the amount of energy released when an electron is added to a neutral atom or molecule to form a negative ion....
is the electron affinityElectron affinityThe Electron affinity of an atom or molecule is defined as the amount of energy released when an electron is added to a neutral atom or molecule to form a negative ion....
of non-metal atom X (fluorine) is the lattice energyLattice energyThe lattice energy of an ionic solid is a measure of the strength of bonds in that ionic compound. It is usually defined as the enthalpy of formation of the ionic compound from gaseous ions and as such is invariably exothermic. Lattice energy may also be defined as the energy required to completely...
is the lattice energyLattice energyThe lattice energy of an ionic solid is a measure of the strength of bonds in that ionic compound. It is usually defined as the enthalpy of formation of the ionic compound from gaseous ions and as such is invariably exothermic. Lattice energy may also be defined as the energy required to completely...
(defined as exothermic here)
The net enthalpy of formation and the first four of the five energies can be determined experimentally, but the lattice energy cannot be measured directly. Instead, the lattice energy is calculated by subtracting the other four energies in the Born–Haber cycle from the net enthalpy of formation.
The word cycle refers to the fact that one can also equate to zero the total enthalpy change for a cyclic process, starting and ending with LiF(s) in the example. This leads to
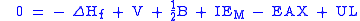
which is equivalent to the previous equation.

