
Lactol
Encyclopedia
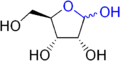
Hemiacetal
Hemiacetals and hemiketals are compounds that are derived from aldehydes and ketones respectively. The Greek word hèmi means half...
or a hemiketal.
The compound is formed by the intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
of a hydroxyl group to the carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group of an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
or a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
.
A lactol is often found as an equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
mixture with the corresponding hydroxyaldehyde. The equilibrium can favor either direction depending on ring size and other conformational effects.
The lactol functional group is prevalent in nature as component of aldose
Aldose
An aldose is a monosaccharide that contains only one aldehyde group per molecule. The chemical formula takes the form Cnn. The simplest possible aldose is the diose glycolaldehyde, which only contains two carbon atoms....
sugars.
Chemical reactivity
Lactols can participate in a variety of chemical reactions including:- OxidationRedoxRedox reactions describe all chemical reactions in which atoms have their oxidation state changed....
to form lactoneLactoneIn chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
s - Reaction with alcoholAlcoholIn chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s to form acetalAcetalAn acetal is a molecule with two single-bonded oxygen atoms attached to the same carbon atom.Traditional usages distinguish ketals from acetals...
s- The reaction of sugars with alcohols or other nucleophileNucleophileA nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s leads to the formation of glycosideGlycosideIn chemistry, a glycoside is a molecule in which a sugar is bound to a non-carbohydrate moiety, usually a small organic molecule. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme...
s
- The reaction of sugars with alcohols or other nucleophile
- ReductionRedoxRedox reactions describe all chemical reactions in which atoms have their oxidation state changed....
(deoxygenation) to form cyclic etherEtherEthers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
s


