
Koenigs-Knorr reaction
Encyclopedia
The Koenigs–Knorr reaction in organic chemistry
is the substitution reaction
of a glycosyl
halide
with an alcohol
to give a glycoside
. It is one of the oldest and simplest glycosylation reactions. It is named after Wilhelm Koenigs (1851–1906), student of von Bayer and fellow student with Hermann Emil Fischer
, and Edward Knorr, student of Koenigs.
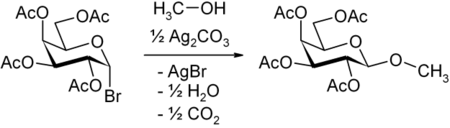 In its original form, Koenigs and Knorr treated acetobromoglucose with alcohols in the presence of silver carbonate
In its original form, Koenigs and Knorr treated acetobromoglucose with alcohols in the presence of silver carbonate
. Shortly afterwards Fischer and Armstrong reported very similar findings.
In the above example, the stereochemical outcome is determined by the presence of the neighboring group at C2 that lends anchimeric assistance, resulting in the formation of a 1,2-trans stereochemical arrangement. Esters (e.g. acetyl, benzoyl, pivalyl) generally provide good anchimeric assistance, whereas ether
s (e.g. benzyl
, methyl etc.) do not, leading to mixtures of stereoisomers.
Generally, the Koenigs–Knorr reaction refers to the use of glycosyl chlorides, bromides and more recently iodides as glycosyl donors.
The Koenigs–Knorr reaction can be performed with alternative promoters such as various heavy metal salts including mercuric bromide/mercuric oxide, mercuric cyanide and silver triflate. When mercury salts are used, the reaction is normally called the Helferich method.
Other glycosidation methods are Fischer glycosidation
, use of glycosyl acetates, thioglycosides, glycosyl trichloroacetimidates, glycosyl fluorides or n-pentenyl glycosides as glycosyl donor
s, or intramolecular aglycon delivery
.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
is the substitution reaction
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
of a glycosyl
Glycosyl
A glycosyl group is a univalent free radical or substituent structure obtained by removing the hemiacetal hydroxyl group from the cyclic form of a monosaccharide and, by extension, of a lower oligosaccharide....
halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
with an alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
to give a glycoside
Glycoside
In chemistry, a glycoside is a molecule in which a sugar is bound to a non-carbohydrate moiety, usually a small organic molecule. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme...
. It is one of the oldest and simplest glycosylation reactions. It is named after Wilhelm Koenigs (1851–1906), student of von Bayer and fellow student with Hermann Emil Fischer
Hermann Emil Fischer
Hermann Emil Fischer, Emil Fischer was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He developed the Fischer projection, a symbolic way of drawing asymmetric carbon atoms.-Early years:Fischer was born in Euskirchen, near Cologne,...
, and Edward Knorr, student of Koenigs.

Silver carbonate
Silver carbonate is the chemical compound with the formula Ag2CO3. Silver carbonate is yellow but typical samples are grayish due to the presence of elemental silver. It is poorly soluble in water, like most transition metal carbonates. Silver carbonate is used as a reagent in organic synthesis...
. Shortly afterwards Fischer and Armstrong reported very similar findings.
In the above example, the stereochemical outcome is determined by the presence of the neighboring group at C2 that lends anchimeric assistance, resulting in the formation of a 1,2-trans stereochemical arrangement. Esters (e.g. acetyl, benzoyl, pivalyl) generally provide good anchimeric assistance, whereas ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
s (e.g. benzyl
Benzyl
In organic chemistry, benzyl is the term used to describe the substituent or molecular fragment possessing the structure C6H5CH2-. Benzyl features a benzene ring attached to a CH2 group.-Nomenclature:...
, methyl etc.) do not, leading to mixtures of stereoisomers.
Generally, the Koenigs–Knorr reaction refers to the use of glycosyl chlorides, bromides and more recently iodides as glycosyl donors.
The Koenigs–Knorr reaction can be performed with alternative promoters such as various heavy metal salts including mercuric bromide/mercuric oxide, mercuric cyanide and silver triflate. When mercury salts are used, the reaction is normally called the Helferich method.
Other glycosidation methods are Fischer glycosidation
Fischer glycosidation
Fischer glycosidation refers to the formation of a glycoside by the reaction of an aldose or ketose with an alcohol in the presence of an acid catalyst...
, use of glycosyl acetates, thioglycosides, glycosyl trichloroacetimidates, glycosyl fluorides or n-pentenyl glycosides as glycosyl donor
Glycosyl donor
A glycosyl donor is a carbohydrate mono- or oligosaccharide that will react with a suitable glycosyl acceptor to form a new glycosidic bond. By convention, the donor is the member of this pair that contains the resulting anomeric carbon of the new glycosidic bond...
s, or intramolecular aglycon delivery
Intramolecular aglycon delivery
Intramolecular aglycon delivery is a synthetic strategy for the construction of glycans. This methodology is generally applied to the formation of difficult glycosidic linkage.-Introduction:...
.

