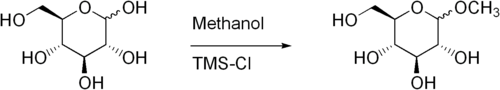
Fischer glycosidation
Encyclopedia
Fischer glycosidation refers to the formation of a glycoside by the reaction of an aldose
or ketose
with an alcohol
in the presence of an acid catalyst. The reaction is named after the German chemist, Emil Hermann Fischer, winner of the Nobel Prize in chemistry, 1902, who developed this method between 1893 and 1895.
Commonly, the reaction is performed using a solution or suspension of the carbohydrate
in the alcohol as the solvent. The carbohydrate is usually completely unprotected. The Fischer glycosidation reaction is an equilibrium process and can lead to a mixture of ring size isomers, and anomers, plus in some cases, small amounts of acyclic forms. With hexoses, short reactions times usually lead to furanose ring forms, and longer reaction times lead to pyranose forms. With long reaction times the most thermodynamically stable product will result which, owing to the anomeric effect
, is usually the alpha anomer.
Aldose
An aldose is a monosaccharide that contains only one aldehyde group per molecule. The chemical formula takes the form Cnn. The simplest possible aldose is the diose glycolaldehyde, which only contains two carbon atoms....
or ketose
Ketose
A ketose is a sugar containing one ketone group per molecule.With 3 carbon atoms, dihydroxyacetone is the simplest of all ketoses and is the only one having no optical activity. Ketoses can isomerize into an aldose when the carbonyl group is located at the end of the molecule...
with an alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
in the presence of an acid catalyst. The reaction is named after the German chemist, Emil Hermann Fischer, winner of the Nobel Prize in chemistry, 1902, who developed this method between 1893 and 1895.
Commonly, the reaction is performed using a solution or suspension of the carbohydrate
Carbohydrate
A carbohydrate is an organic compound with the empirical formula ; that is, consists only of carbon, hydrogen, and oxygen, with a hydrogen:oxygen atom ratio of 2:1 . However, there are exceptions to this. One common example would be deoxyribose, a component of DNA, which has the empirical...
in the alcohol as the solvent. The carbohydrate is usually completely unprotected. The Fischer glycosidation reaction is an equilibrium process and can lead to a mixture of ring size isomers, and anomers, plus in some cases, small amounts of acyclic forms. With hexoses, short reactions times usually lead to furanose ring forms, and longer reaction times lead to pyranose forms. With long reaction times the most thermodynamically stable product will result which, owing to the anomeric effect
Anomeric effect
In organic chemistry, the anomeric effect or Edward-Lemieux effect is a stereoelectronic effect that describes the tendency of heteroatomic substituents adjacent to a heteroatom within a cyclohexane ring to prefer the axial orientation instead of the less hindered equatorial orientation that would...
, is usually the alpha anomer.