
Iron group
Encyclopedia
In chemistry
and physics
, the iron group refers to elements
that are in some way related to iron
. These elements are relatively abundant both on Earth and elsewhere in the universe. The term is ambiguous in different contexts, and almost obsolete in chemistry.
referred to the elements iron, cobalt
and nickel
, that is the first row of group VIII (or VIIIB) under the old numbering system. These metals, and the platinum group
immediately below them, were set aside from the other elements as they show obvious similarities among themselves in their chemistry, but are not obviously related to any of the other groups.
The similarities in chemistry along what is now known as the first row of the transition metals were noted by Adolph Strecker
in 1859. Newlands'
"octaves" (1865) were harshly criticized for separating iron from cobalt and nickel. Mendeleev
stressed that groups of "chemically analogous elements" could have similar atomic weight
s as well as atomic weights which increase by equal increments, both in his original 1869 paper and his 1889 Faraday Lecture.
The main cations in the iron group are iron itself (Fe2+ and Fe3+), aluminium
(Al3+) and chromium
(Cr3+). If manganese
is present in the sample, a small amount of hydrated manganese dioxide is often precipitated with the iron group hydroxides. Less common cations which are precipitated with the iron group include beryllium
, titanium
, zirconium
, vanadium
, uranium
, thorium
and cerium
.
to nickel
which are substantially more abundant in the universe than those that come after them – or immediately before them – in order of atomic number
. The study of the abundances of iron group elements relative to other elements in star
s and supernova
e allows the refinement of models of stellar evolution
.
The explanation for this relative abundance can be found in the process of nucleosynthesis
in certain stars, specifically those of about 8–11 Solar mass
es. At the end of their lives, once other fuels have been exhausted, such stars can under a brief phase of "silicon burning
". This involves the sequential addition of helium
nucli
(an "alpha process") to the heavier elements present in the star, starting from :
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ || In lighter stars, with less gravitational pressure, the alpha process is much slower and effectively stops at this stage as titanium-44 is unstable with respect to beta decay (t½ = 60.0(11) years).
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|}
All of these nuclear reactions are exothermic, that is they release energy: the energy that is released partially offsets the gravitational contraction of the star. However, the series ends at , as the next reaction in the series,
is endothermic. With no further source of energy to support itself, the core of the star collapses on itself while the outer regions are blown off in a Type II
supernova
.
Nickel-56 is unstable with respect to beta decay
, and the final stable product of silicon burning is .
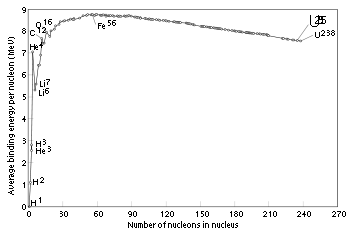
It is often incorrectly assumed that iron-56 is the most stable of all the nuclides. This is not quite true: and have slightly higher binding energies per nucleon
– that is, they are slightly more stable as nuclides – as can be seen from the table on the right. However, there are no rapid nucleosynthetic routes to these nuclides. There are several stable nuclides of elements from chromium to nickel around the top of the stability curve, accounting for their relative abundance in the universe. The nuclides which are not on the direct alpha-process pathway are formed by the so-called S-process
, the capture of slow neutron
s within the star.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
and physics
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
, the iron group refers to elements
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
that are in some way related to iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
. These elements are relatively abundant both on Earth and elsewhere in the universe. The term is ambiguous in different contexts, and almost obsolete in chemistry.
Periodic table
The iron group in the periodic tablePeriodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
referred to the elements iron, cobalt
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
and nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
, that is the first row of group VIII (or VIIIB) under the old numbering system. These metals, and the platinum group
Platinum group
The platinum group metals is a term used sometimes to collectively refer to six metallic elements clustered together in the periodic table.These elements are all transition metals, lying in the d-block .The six...
immediately below them, were set aside from the other elements as they show obvious similarities among themselves in their chemistry, but are not obviously related to any of the other groups.
The similarities in chemistry along what is now known as the first row of the transition metals were noted by Adolph Strecker
Adolph Strecker
Adolph Strecker was a German chemist who is remembered primarily for his work with amino acids.- Life and work :...
in 1859. Newlands'
John Alexander Reina Newlands
John Alexander Reina Newlands was an English chemist who invented the Periodic Table.Newlands was born in London and was the son of a scottish Presbyterian minister and his Italian wife....
"octaves" (1865) were harshly criticized for separating iron from cobalt and nickel. Mendeleev
Dmitri Mendeleev
Dmitri Ivanovich Mendeleev , was a Russian chemist and inventor. He is credited as being the creator of the first version of the periodic table of elements...
stressed that groups of "chemically analogous elements" could have similar atomic weight
Atomic weight
Atomic weight is a dimensionless physical quantity, the ratio of the average mass of atoms of an element to 1/12 of the mass of an atom of carbon-12...
s as well as atomic weights which increase by equal increments, both in his original 1869 paper and his 1889 Faraday Lecture.
Analytical chemistry
In the traditional methods of qualitative inorganic analysis, the iron group consists of those cations which- have soluble chlorideChlorideThe chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
s; and - are not precipitated as sulfideSulfideA sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
s by hydrogen sulfideHydrogen sulfideHydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
in acidAcidAn acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
ic conditions; - are precipitated as hydroxideHydroxideHydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
s at around pH 10 (or less) in the presence of ammoniaAmmoniaAmmonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
.
The main cations in the iron group are iron itself (Fe2+ and Fe3+), aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
(Al3+) and chromium
Chromium
Chromium is a chemical element which has the symbol Cr and atomic number 24. It is the first element in Group 6. It is a steely-gray, lustrous, hard metal that takes a high polish and has a high melting point. It is also odorless, tasteless, and malleable...
(Cr3+). If manganese
Manganese
Manganese is a chemical element, designated by the symbol Mn. It has the atomic number 25. It is found as a free element in nature , and in many minerals...
is present in the sample, a small amount of hydrated manganese dioxide is often precipitated with the iron group hydroxides. Less common cations which are precipitated with the iron group include beryllium
Beryllium
Beryllium is the chemical element with the symbol Be and atomic number 4. It is a divalent element which occurs naturally only in combination with other elements in minerals. Notable gemstones which contain beryllium include beryl and chrysoberyl...
, titanium
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
, zirconium
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
, vanadium
Vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery gray, ductile and malleable transition metal. The formation of an oxide layer stabilizes the metal against oxidation. The element is found only in chemically combined form in nature...
, uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
, thorium
Thorium
Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder....
and cerium
Cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. It is a soft, silvery, ductile metal which easily oxidizes in air. Cerium was named after the dwarf planet . Cerium is the most abundant of the rare earth elements, making up about 0.0046% of the Earth's crust by weight...
.
Astrophysics
The iron group in astrophysics is the group of elements from chromiumChromium
Chromium is a chemical element which has the symbol Cr and atomic number 24. It is the first element in Group 6. It is a steely-gray, lustrous, hard metal that takes a high polish and has a high melting point. It is also odorless, tasteless, and malleable...
to nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
which are substantially more abundant in the universe than those that come after them – or immediately before them – in order of atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
. The study of the abundances of iron group elements relative to other elements in star
Star
A star is a massive, luminous sphere of plasma held together by gravity. At the end of its lifetime, a star can also contain a proportion of degenerate matter. The nearest star to Earth is the Sun, which is the source of most of the energy on Earth...
s and supernova
Supernova
A supernova is a stellar explosion that is more energetic than a nova. It is pronounced with the plural supernovae or supernovas. Supernovae are extremely luminous and cause a burst of radiation that often briefly outshines an entire galaxy, before fading from view over several weeks or months...
e allows the refinement of models of stellar evolution
Stellar evolution
Stellar evolution is the process by which a star undergoes a sequence of radical changes during its lifetime. Depending on the mass of the star, this lifetime ranges from only a few million years to trillions of years .Stellar evolution is not studied by observing the life of a single...
.
The explanation for this relative abundance can be found in the process of nucleosynthesis
Nucleosynthesis
Nucleosynthesis is the process of creating new atomic nuclei from pre-existing nucleons . It is thought that the primordial nucleons themselves were formed from the quark–gluon plasma from the Big Bang as it cooled below two trillion degrees...
in certain stars, specifically those of about 8–11 Solar mass
Solar mass
The solar mass , , is a standard unit of mass in astronomy, used to indicate the masses of other stars and galaxies...
es. At the end of their lives, once other fuels have been exhausted, such stars can under a brief phase of "silicon burning
Silicon burning process
In astrophysics, silicon burning is a very brief sequence of nuclear fusion reactions that occur in massive stars with a minimum of about 8–11 solar masses. Silicon burning is the final stage of fusion for massive stars that have run out of the fuels that power them for their long lives in the main...
". This involves the sequential addition of helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
nucli
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
(an "alpha process") to the heavier elements present in the star, starting from :
- {| border="0"
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ || In lighter stars, with less gravitational pressure, the alpha process is much slower and effectively stops at this stage as titanium-44 is unstable with respect to beta decay (t½ = 60.0(11) years).
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|- style="height:2em;"
| ||+ || ||→ ||
|}
All of these nuclear reactions are exothermic, that is they release energy: the energy that is released partially offsets the gravitational contraction of the star. However, the series ends at , as the next reaction in the series,
| → |
is endothermic. With no further source of energy to support itself, the core of the star collapses on itself while the outer regions are blown off in a Type II
Type II supernova
A Type II supernova results from the rapid collapse and violent explosion of a massive star. A star must have at least 9 times, and no more than 40–50 times the mass of the Sun for this type of explosion. It is distinguished from other types of supernova by the presence of hydrogen in its spectrum...
supernova
Supernova
A supernova is a stellar explosion that is more energetic than a nova. It is pronounced with the plural supernovae or supernovas. Supernovae are extremely luminous and cause a burst of radiation that often briefly outshines an entire galaxy, before fading from view over several weeks or months...
.
Nickel-56 is unstable with respect to beta decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
, and the final stable product of silicon burning is .
| → | β+ Positron The positron or antielectron is the antiparticle or the antimatter counterpart of the electron. The positron has an electric charge of +1e, a spin of ½, and has the same mass as an electron... |
t½ = 6.075(10) d | ||
| → | β+ | t½ = 77.233(27) d |
Nuclide stability
| Nuclide mass | Mass defect | Binding energy per nucleon |
|
|---|---|---|---|
| 61.9283451(6) u | 0.5700031(6) u | 8.563872(10) MeV | |
| 57.9332756(8) u | 0.5331899(8) u | 8.563158(12) MeV | |
| 55.9349375(7) u | 0.5141981(7) u | 8.553080(12) MeV | |
It is often incorrectly assumed that iron-56 is the most stable of all the nuclides. This is not quite true: and have slightly higher binding energies per nucleon
Binding energy
Binding energy is the mechanical energy required to disassemble a whole into separate parts. A bound system typically has a lower potential energy than its constituent parts; this is what keeps the system together—often this means that energy is released upon the creation of a bound state...
– that is, they are slightly more stable as nuclides – as can be seen from the table on the right. However, there are no rapid nucleosynthetic routes to these nuclides. There are several stable nuclides of elements from chromium to nickel around the top of the stability curve, accounting for their relative abundance in the universe. The nuclides which are not on the direct alpha-process pathway are formed by the so-called S-process
S-process
The S-process or slow-neutron-capture-process is a nucleosynthesis process that occurs at relatively low neutron density and intermediate temperature conditions in stars. Under these conditions the rate of neutron capture by atomic nuclei is slow relative to the rate of radioactive beta-minus decay...
, the capture of slow neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
s within the star.

See also
- Sun#Singly ionized iron group elements
- S-processS-processThe S-process or slow-neutron-capture-process is a nucleosynthesis process that occurs at relatively low neutron density and intermediate temperature conditions in stars. Under these conditions the rate of neutron capture by atomic nuclei is slow relative to the rate of radioactive beta-minus decay...
- Silicon burning processSilicon burning processIn astrophysics, silicon burning is a very brief sequence of nuclear fusion reactions that occur in massive stars with a minimum of about 8–11 solar masses. Silicon burning is the final stage of fusion for massive stars that have run out of the fuels that power them for their long lives in the main...
- Abundance of the chemical elementsAbundance of the chemical elementsThe abundance of a chemical element measures how relatively common the element is, or how much of the element is present in a given environment by comparison to all other elements...

