
Inosinic acid
Encyclopedia
Inosinic acid or inosine monophosphate (IMP) is a nucleotide
monophosphate. Inosinic acid is important in metabolism
. It is the ribonucleotide
of hypoxanthine
and the first nucleotide formed during the synthesis of purine
. It is formed by the deamination
of adenosine monophosphate
, and is hydrolysed
from inosine
. IMP is an intermediate ribonucleoside monophosphate in purine metabolism
.
Important derivatives of inosinic acid include purine nucleotides found in nucleic acid
s and adenosine triphosphate
, which is used to store chemical energy
in muscle
and other tissues.
In the food industry, inosinic acid and its salts such as disodium inosinate
are used as flavour enhancer
s.
(PRPP). In the first step, an amino group given by glutamine is attached at carbon 1 of PRPP. The resulting molecule is 5-phosphoribosylamine, which is highly unstable, with a half-life of 30 seconds at physiologic pH
. 5-Phosphoribosylamine gains an amino acid (glycine
), becoming glycinamide ribonucleotide (GAR). Then, N10-formyltetrahydrofolate (Tetrahydrofolate) transfers a formyl group to glycinamide ribonucleotide to form formyl glycinamide ribonucleotide (FGAR).
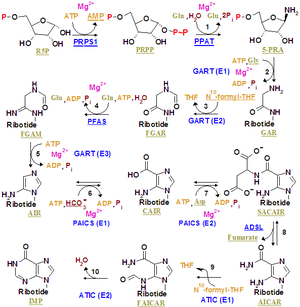 Using an ATP
Using an ATP
molecule, glutamine
donates an ammonia
molecule which is added to the compound forming formylglycinamidine ribonucleotide. Another ATP molecule causes an intermolecular reaction that produces an imidazole
ring (5-aminoimidazole ribonucleotide).
The next step of the pathway is adding bicarbonate
to make carboxyaminoimidazole ribonucleotide by using ATP (it only happens in fungi and bacteria
; high eukaryotes simply add CO2 to form the ribonucleotide). Then, the imidazole’s carboxylate group phosphatises and adds aspartate.
As we have just seen, a six-step process links glycine
, formate
, bicarbonate
, glutamine
, and aspartate to lead to an intermediate that contains almost all the required atoms to synthesize a purine ring. This intermediate removes fumarate, and a second formyl group from THF is added. The compound gets cycled and forms inosinate after a sort of intermolecular reactions. Inosinate is the first intermediate in this synthesis pathway to have a whole purine ring.
Enzymes taking part in IMP synthesis constitute a multienzyme complex in the cell. Evidences demonstrate that there are multifunctional enzymes, and some of them catalyze non-sequential steps in the pathway.
or GMP
. Both compounds are RNA
nucleotides.
AMP differs from inosinate by the replacement of IMP's carbon-6 carbonyl with an amino group. The interconversion of AMP and IMP occurs as part of the purine nucleotide cycle
.
GMP is formed by the inosinate oxidation to xanthylate (XMP), and afterwards adds an amino group on carbon 2. Hydrogen acceptor on inosinate oxidation is NAD+. Finally, carbon 2 gains the amino group by spending an ATP molecule (which becomes AMP+2Pi).
While AMP synthesis requires GTP, GMP synthesis uses ATP. That difference offers an important regulation possibility.
E 631, dipotassium inosinate E 632, and dicalcium inosinate E 633.
These three compounds are used as flavour enhancers with a comparatively high effectiveness. They are mostly used in soups, sauces, and seasonings for the intensification and balance of meat taste.
Nucleotide
Nucleotides are molecules that, when joined together, make up the structural units of RNA and DNA. In addition, nucleotides participate in cellular signaling , and are incorporated into important cofactors of enzymatic reactions...
monophosphate. Inosinic acid is important in metabolism
Metabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
. It is the ribonucleotide
Ribonucleotide
A ribonucleotide or ribotide is a nucleotide in which a purine or pyrimidine base is linked to a ribose molecule and exactly one phosphate group. In living organisms the most common bases for ribonucleotides are adenine , guanine , cytosine , or uracil ....
of hypoxanthine
Hypoxanthine
Hypoxanthine is a naturally occurring purine derivative. It is occasionally found as a constituent of nucleic acids where it is present in the anticodon of tRNA in the form of its nucleoside inosine. It has a tautomer known as 6-Hydroxypurine. Hypoxanthine is a necessary additive in certain cell,...
and the first nucleotide formed during the synthesis of purine
Purine
A purine is a heterocyclic aromatic organic compound, consisting of a pyrimidine ring fused to an imidazole ring. Purines, including substituted purines and their tautomers, are the most widely distributed kind of nitrogen-containing heterocycle in nature....
. It is formed by the deamination
Deamination
Deamination is the removal of an amine group from a molecule. Enzymes which catalyse this reaction are called deaminases.In the human body, deamination takes place primarily in the liver, however glutamate is also deaminated in the kidneys. Deamination is the process by which amino acids are...
of adenosine monophosphate
Adenosine monophosphate
Adenosine monophosphate , also known as 5'-adenylic acid, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid and the nucleoside adenosine. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine...
, and is hydrolysed
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
from inosine
Inosine
Inosine is a nucleoside that is formed when hypoxanthine is attached to a ribose ring via a β-N9-glycosidic bond....
. IMP is an intermediate ribonucleoside monophosphate in purine metabolism
Purine metabolism
-Biosynthesis:Purines are biologically synthesized as nucleotides and in particular as ribotides, i.e. bases attached to ribose 5-phosphate. A key regulatory step is the production of 5-phospho-α-D-ribosyl 1-pyrophosphate by PRPP synthetase, which is activated by inorganic phosphate and...
.
Important derivatives of inosinic acid include purine nucleotides found in nucleic acid
Nucleic acid
Nucleic acids are biological molecules essential for life, and include DNA and RNA . Together with proteins, nucleic acids make up the most important macromolecules; each is found in abundance in all living things, where they function in encoding, transmitting and expressing genetic information...
s and adenosine triphosphate
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
, which is used to store chemical energy
Chemical energy
Chemical energy is the potential of a chemical substance to undergo a transformation through a chemical reaction or, to transform other chemical substances...
in muscle
Muscle
Muscle is a contractile tissue of animals and is derived from the mesodermal layer of embryonic germ cells. Muscle cells contain contractile filaments that move past each other and change the size of the cell. They are classified as skeletal, cardiac, or smooth muscles. Their function is to...
and other tissues.
In the food industry, inosinic acid and its salts such as disodium inosinate
Disodium inosinate
Disodium inosinate is the disodium salt of inosinic acid with the chemical formula C10H11N4Na2O8P. It is used as a food additive and often found in instant noodles, potato chips, and a variety of other snacks.-Use as a food additive:...
are used as flavour enhancer
Flavour enhancer
Flavour enhancers are food additives commonly added to food and designed to enhance the existing flavours of products. In western cultures, the 5th taste or umami went unrecognized for a long time. It was believed that flavour enhancers did not add any new taste of their own...
s.
Inosinate synthesis
The inosinate synthesis is complex, beginning with a 5-phosphoribosyl-1-pyrophosphatePhosphoribosyl pyrophosphate
Phosphoribosyl pyrophosphate is a pentosephosphate.It is formed from ribose 5-phosphate by the enzyme ribose-phosphate diphosphokinase.It plays a role in transferring phospho-ribose groups in several reactions:...
(PRPP). In the first step, an amino group given by glutamine is attached at carbon 1 of PRPP. The resulting molecule is 5-phosphoribosylamine, which is highly unstable, with a half-life of 30 seconds at physiologic pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
. 5-Phosphoribosylamine gains an amino acid (glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
), becoming glycinamide ribonucleotide (GAR). Then, N10-formyltetrahydrofolate (Tetrahydrofolate) transfers a formyl group to glycinamide ribonucleotide to form formyl glycinamide ribonucleotide (FGAR).

Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
molecule, glutamine
Glutamine
Glutamine is one of the 20 amino acids encoded by the standard genetic code. It is not recognized as an essential amino acid but may become conditionally essential in certain situations, including intensive athletic training or certain gastrointestinal disorders...
donates an ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
molecule which is added to the compound forming formylglycinamidine ribonucleotide. Another ATP molecule causes an intermolecular reaction that produces an imidazole
Imidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
ring (5-aminoimidazole ribonucleotide).
The next step of the pathway is adding bicarbonate
Bicarbonate
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
to make carboxyaminoimidazole ribonucleotide by using ATP (it only happens in fungi and bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
; high eukaryotes simply add CO2 to form the ribonucleotide). Then, the imidazole’s carboxylate group phosphatises and adds aspartate.
As we have just seen, a six-step process links glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
, formate
Formate
Formate or methanoate is the ion CHOO− or HCOO− . It is the simplest carboxylate anion. It is produced in large amounts in the hepatic mitochondria of embryonic cells and in cancer cells by the folate cycle Formate or methanoate is the ion CHOO− or HCOO− (formic acid minus one hydrogen ion). It...
, bicarbonate
Bicarbonate
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
, glutamine
Glutamine
Glutamine is one of the 20 amino acids encoded by the standard genetic code. It is not recognized as an essential amino acid but may become conditionally essential in certain situations, including intensive athletic training or certain gastrointestinal disorders...
, and aspartate to lead to an intermediate that contains almost all the required atoms to synthesize a purine ring. This intermediate removes fumarate, and a second formyl group from THF is added. The compound gets cycled and forms inosinate after a sort of intermolecular reactions. Inosinate is the first intermediate in this synthesis pathway to have a whole purine ring.
Enzymes taking part in IMP synthesis constitute a multienzyme complex in the cell. Evidences demonstrate that there are multifunctional enzymes, and some of them catalyze non-sequential steps in the pathway.
Adenylate (AMP) and guanylate (GMP) take form from inosinate
Within a few steps inosinate becomes AMPAdenosine monophosphate
Adenosine monophosphate , also known as 5'-adenylic acid, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid and the nucleoside adenosine. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine...
or GMP
Guanosine monophosphate
Guanosine monophosphate, also known as 5'-guanidylic acid or guanylic acid and abbreviated GMP, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid with the nucleoside guanosine. GMP consists of the phosphate group, the pentose sugar ribose, and the nucleobase...
. Both compounds are RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
nucleotides.
AMP differs from inosinate by the replacement of IMP's carbon-6 carbonyl with an amino group. The interconversion of AMP and IMP occurs as part of the purine nucleotide cycle
Purine nucleotide cycle
The Purine Nucleotide Cycle is a metabolic pathway in which fumarate is generated from aspartate in order to increase the concentration of Krebs cycle intermediates. The pathway was first described by John Lowenstein, who demonstrated its role in increasing the rate of oxidative phosphorylation in...
.
GMP is formed by the inosinate oxidation to xanthylate (XMP), and afterwards adds an amino group on carbon 2. Hydrogen acceptor on inosinate oxidation is NAD+. Finally, carbon 2 gains the amino group by spending an ATP molecule (which becomes AMP+2Pi).
While AMP synthesis requires GTP, GMP synthesis uses ATP. That difference offers an important regulation possibility.
Inosinate takes part on the regulation of purine nucleotides biosynthesis
Inosinate and many other molecules inhibit the synthesis of 5-phosphorybosilamine from 5-phosphoribosyl-1-pyrophosphate (PRPP), disabling the enzyme that catalyzes the reaction: glutamine-5-phosphoribosyl-1-pyrophosphate-amidotransferase. In other words, when levels of inosinate are high, glutamine-5-phosphoribosyl-1-pyrophosphate-amidotransferase is inhibited, and, as a consequence, inosinate levels decrease. Also, as a result, adenylate and guanylate are not produced, which means that RNA synthesis cannot be completed because of the lack of these two important RNA nucleotides.Applications
Inosinate can be manufactured into various compounds with the aid of genetically modified organisms: disodium inosinateDisodium inosinate
Disodium inosinate is the disodium salt of inosinic acid with the chemical formula C10H11N4Na2O8P. It is used as a food additive and often found in instant noodles, potato chips, and a variety of other snacks.-Use as a food additive:...
E 631, dipotassium inosinate E 632, and dicalcium inosinate E 633.
These three compounds are used as flavour enhancers with a comparatively high effectiveness. They are mostly used in soups, sauces, and seasonings for the intensification and balance of meat taste.

