Homologation reaction
Encyclopedia
A homologation reaction, also known as homologization, is any chemical reaction
that converts the reactant into the next member of the homologous series
. A Homologous series
is a group of compounds that differ by a constant unit, generally a (-CH2-) group. The reactants undergo a homologation when the number of a repeated structural unit in the molecules is increased. The most common homologation reactions increase the number of methylene
(-CH2-) units in saturated chain within the molecule. For example the reaction of aldehyde
s or ketone
s with diazomethane
or methoxymethylenetriphenylphosphine
to give the next homologue in the series.
Examples of homologation reactions include:
Some reactions increase the chain length by more than one unit. For example, the following are considered two-carbon homologation reactions.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
that converts the reactant into the next member of the homologous series
Homologous series
In chemistry, a homologous series is a series of compounds with a similar general formula, possessing similar chemical properties due to the presence of the same functional group, and showing a gradation in physical properties as a result of increase in molecular size and mass...
. A Homologous series
Homologous series
In chemistry, a homologous series is a series of compounds with a similar general formula, possessing similar chemical properties due to the presence of the same functional group, and showing a gradation in physical properties as a result of increase in molecular size and mass...
is a group of compounds that differ by a constant unit, generally a (-CH2-) group. The reactants undergo a homologation when the number of a repeated structural unit in the molecules is increased. The most common homologation reactions increase the number of methylene
Methylene
Methylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
(-CH2-) units in saturated chain within the molecule. For example the reaction of aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s or ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s with diazomethane
Diazomethane
Diazomethane is the chemical compound CH2N2. It is the simplest of diazo compounds. In the pure form at room temperature, it is a extremely sensitive explosive yellow gas, thus it is almost universally used as a solution in diethyl ether...
or methoxymethylenetriphenylphosphine
Methoxymethylenetriphenylphosphine
Methoxymethylenetriphenylphosphine is a Wittig reagent with used as an reagent in the homologization of aldehydes and ketones to extended aldehydes, an organic reaction first reported in 1958 ....
to give the next homologue in the series.
Examples of homologation reactions include:
- Kiliani-Fischer synthesisKiliani-Fischer synthesisThe Kiliani–Fischer synthesis, named for German chemists Heinrich Kiliani and Hermann Emil Fischer, is a method for synthesizing monosaccharides. It proceeds via synthesis and hydrolysis of a cyanohydrin, thus elongating the carbon chain of an aldose by one carbon atom while preserving...
, where an aldoseAldoseAn aldose is a monosaccharide that contains only one aldehyde group per molecule. The chemical formula takes the form Cnn. The simplest possible aldose is the diose glycolaldehyde, which only contains two carbon atoms....
molecule is elongated through a three-step process consisting of:- Nucleophillic addition of cyanide to the carbonyl to form a cyanohydrinCyanohydrinA cyanohydrin is a functional group found in organic compounds. Cyanohydrins have the formula R2CCN, where R is H, alkyl, or aryl. Cyanohydrins are industrially important precursors to carboxylic acids and some amino acids...
- Hydrolysis to form a lactoneLactoneIn chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
- Reduction to form the homologous aldose
- Nucleophillic addition of cyanide to the carbonyl to form a cyanohydrin
- Wittig reactionWittig reactionThe Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
of an aldehyde with methoxymethylenetriphenylphosphineMethoxymethylenetriphenylphosphineMethoxymethylenetriphenylphosphine is a Wittig reagent with used as an reagent in the homologization of aldehydes and ketones to extended aldehydes, an organic reaction first reported in 1958 ....
, which produces a homologous aldehyde. - Arndt–Eistert reaction is a series of chemical reactions designed to convert a carboxylic acid to a higher carboxylic acid homologue (i.e. contains one additional carbon atom)
- Kowalski ester homologationKowalski ester homologationThe Kowalski ester homologation is a chemical reaction for the homologation of esters.This reaction was designed as a safer alternative to the Arndt-Eistert synthesis. The Kowalski reaction is named after its inventor, Conrad J. Kowalski....
, an alternative to the Arndt-Eistert synthesis. Has been used to convert β-amino esters from α-amino esters through an ynolateYnolateYnolates are chemical compounds with a negatively charged oxygen attached to an alkyne functionality. They were first synthesized in 1975 by Schöllkopf and Hoppe via the n-butyllithium fragmentation of 3,4-diphenylisoxazole....
intermediate. - Seyferth–Gilbert homologation in which an aldehyde is converted to a terminal alkyne and then hydrolyzed back to a aldehyde.
Some reactions increase the chain length by more than one unit. For example, the following are considered two-carbon homologation reactions.
Chain reduction
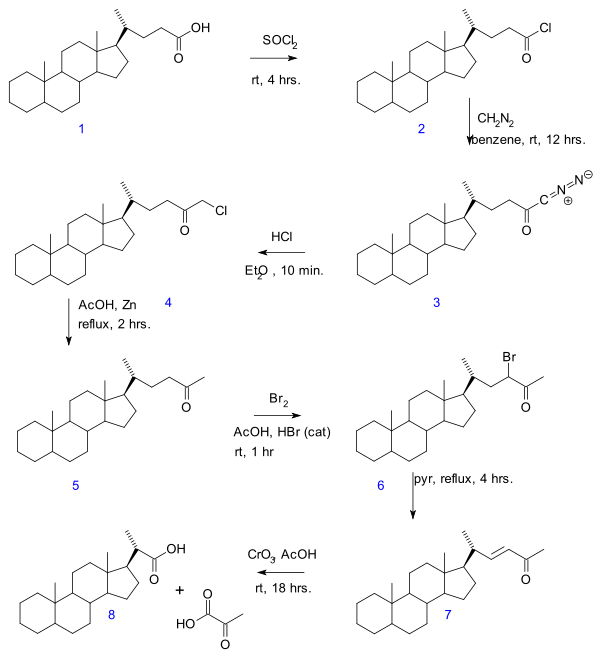
Likewise the chain length can also be reduced:- In the Gallagher-Hollander Degradation (1946) pyruvic acidPyruvic acidPyruvic acid is an organic acid, a ketone, as well as the simplest of the alpha-keto acids. The carboxylate ion of pyruvic acid, CH3COCOO−, is known as pyruvate, and is a key intersection in several metabolic pathways....
is removed from a linear aliphatic carboxylic acidCarboxylic acidCarboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
yielding a new acid with 2 carbon atoms less. The original publication concerns the conversion of bile acidBile acidBile acids are steroid acids found predominantly in the bile of mammals. Bile salts are bile acids compounded with a cation, usually sodium. In humans, the salts of taurocholic acid and glycocholic acid represent approximately eighty percent of all bile salts. The two major bile acids are cholic...
in a series of reactions: acid chloride (2) formation with thionyl chlorideThionyl chlorideThionyl chloride is an inorganic compound with the formula SOCl2. It is a reactive chemical reagent used in chlorination reactions. It is a colorless, distillable liquid at room temperature and pressure that decomposes above 140 °C. Thionyl chloride is sometimes confused with sulfuryl...
, diazoketone formation (3) with diazomethaneDiazomethaneDiazomethane is the chemical compound CH2N2. It is the simplest of diazo compounds. In the pure form at room temperature, it is a extremely sensitive explosive yellow gas, thus it is almost universally used as a solution in diethyl ether...
, chloromethyl ketone formation (4) with hydrochloric acidHydrochloric acidHydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
, organic reduction of chlorine to methylketone (5), ketone halogenationKetone halogenationIn organic chemistry ketone halogenation is a special type of halogenation.The position alpha to the carbonyl group in a ketone is easily halogenated, due to the ability to form an enolate in basic solution, or an enol in acidic solution...
to 6, elimination reactionElimination reactionAn elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
with pyridinePyridinePyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
to enoneEnoneAn enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
7 and finally oxidation with chromium trioxideChromium trioxideChromium trioxide is the inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name.This compound is a dark-red/orange brown solid, which dissolves in water concomitant with hydrolysis...
to bisnorcholanic acid 8.
- In the Hooker reaction (1936) an alkyl chain in a certain naphthoquinoneNaphthoquinoneNaphthoquinone is a class of natural phenols based on the C6-C4 skeleton.1,4-Naphthoquinone can be viewed as derivatives of naphthalene through the replacement of two hydrogen atoms by two ketone groups....
(phenomenon first observed in the compound lapacholLapacholLapachol is a natural organic compound isolated from the lapacho tree . Chemically, it is a derivative of naphthoquinone, related to vitamin K....
) is reduced by one methyleneMethyleneMethylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
unit as carbon dioxideCarbon dioxideCarbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
in each potassium permanganatePotassium permanganatePotassium permanganate is an inorganic chemical compound with the formula KMnO4. It is a salt consisting of K+ and MnO4− ions. Formerly known as permanganate of potash or Condy's crystals, it is a strong oxidizing agent. It dissolves in water to give intensely purple solutions, the...
oxidation.

- Mechanistically oxidation causes ring-cleavage at the alkene group, extrusion of carbon dioxideCarbon dioxideCarbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
in decarboxylationDecarboxylationDecarboxylation is a chemical reaction that releases carbon dioxide . Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carbonation, the addition of CO2 to...
with subsequent ring-closure.