
Henry's law
Encyclopedia
In physics
, Henry's law is one of the gas laws
formulated by William Henry
in 1803. It states that:
An equivalent way of stating the law is that the solubility
of a gas in a liquid at a particular temperature is proportional to the pressure of that gas above the liquid. Henry's law has since been shown to apply for a wide range of dilute solution
s, not merely those of gases.
An everyday example of Henry's law is given by carbonated
soft drink
s. Before the bottle or can is opened, the gas above the drink is almost pure carbon dioxide
at a pressure slightly higher than atmospheric pressure
. The drink itself contains dissolved carbon dioxide. When the bottle or can is opened, some of this gas escapes, giving the characteristic hiss (or "pop" in the case of a champagne bottle). Because the pressure above the liquid is now lower, some of the dissolved carbon dioxide comes out of solution as bubbles. If a glass of the drink is left in the open, the concentration of carbon dioxide in solution will come into equilibrium with the carbon dioxide in the air, and the drink will go "flat". Note that the pressure acting above the drink in the sealed must come from the partial pressure of carbon dioxide. If the gas is only air it would not produce the same effect even if the pressure value is the same.
A slightly more exotic example of Henry's law is in decompression
and decompression sickness
of divers
.
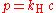
where p is the partial pressure
of the solute
in the gas above the solution, c is the concentration
of the solute and kH is a constant with the dimensions
of pressure divided by concentration. The constant, known as the Henry's law constant, depends on the solute, the solvent and the temperature.
Some values for kH for gases dissolved in water at 298 K
include:
There are other forms of Henry's Law, each of which defines the constant kH differently and requires different dimensional units. In particular, the "concentration" of the solute in solution may also be expressed as a mole fraction or as a molality.
where:
As can be seen by comparing the equations in the above table, the Henry's law constant kH,pc is simply the inverse of the constant kH,cp. Since all kH may be referred to as Henry's law constants, readers of the technical literature must be quite careful to note which version of the Henry's Law equation is being used.
It should also be noted the Henry's Law is a limiting law that only applies for sufficiently dilute solutions. The range of concentrations in which it applies becomes narrower the more the system diverges from ideal behavior. Roughly speaking, that is the more chemically different the solute is from the solvent.
It also only applies simply for solutions where the solvent does not react chemically
with the gas being dissolved. A common example of a gas that does react with the solvent is carbon dioxide
, which forms carbonic acid
(H2CO3) to a certain degree with water.

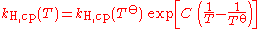
where
This equation is only an approximation, and should be used only when no better, experimentally-derived formula is known for a given gas.
The following table lists some values for constant C (in kelvins) in the equation above:
Because solubility of permanent gases usually decreases with increasing temperature at around the room temperature, the partial pressure a given gas concentration has in liquid must increase. While heating water (saturated with nitrogen) from 25 °C to 95 °C the solubility will decrease to about 43% of its initial value. This can be verified when heating water in a pot: small bubbles evolve and rise, long before the water reaches boiling temperature. Similarly, carbon dioxide from a carbonated
drink escapes much faster when the drink is not cooled because the required partial pressure of CO2 to achieve the same solubility increases in higher temperatures. Partial pressure of CO2 in the gas phase in equilibrium with seawater doubles with every 16 K increase in temperature.
The constant C may be regarded as:
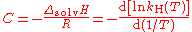
where
The solubility of gases does not always decrease with increasing temperature. For aqueous solutions, the Henry-law constant usually goes through a maximum (i.e., the solubility goes through a minimum). For most permanent gases, the minimum is below 120 °C. It is often observed that the smaller the gas molecule (and the lower the gas solubility in water), then the lower the temperature of the maximum of the Henry-law constant. Thus, the maximum is about 30 °C for helium, 92 to 93 °C for argon, nitrogen and oxygen, and 114 °C for xenon.
, a version of Henry's law applies to the solubility of a noble gas
in contact with silicate
melt. One equation used is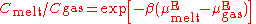
where:
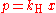
This can be compared with Raoult's law: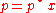
where p* is the vapor pressure of the pure component.
At first sight, Raoult's law appears to be a special case of Henry's law where kH = p*. This is true for pairs of closely related substances, such as benzene
and toluene
, which obey Raoult's law over the entire composition range: such mixtures are called "ideal mixtures".
The general case is that both laws are limit laws
, and they apply at opposite ends of the composition range. The vapor pressure of the component in large excess, such as the solvent for a dilute solution, is proportional to its mole fraction, and the constant of proportionality is the vapor pressure of the pure substance (Raoult's law). The vapor pressure of the solute is also proportional to the solute's mole fraction, but the constant of proportionality is different and must be determined experimentally (Henry's law). In mathematical terms:
Raoult's law can also be related to non-gas solutes.
or even sodium chloride
. In these cases, it is necessary to state the law in terms of chemical potential
s. For a solute in an ideal dilute solution, the chemical potential depends on the concentration: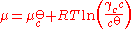 , where
, where 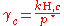 for a volatile solute; c
for a volatile solute; co = 1 mol/L.
For non-ideal solutions, the activity coefficient
γc depends on the concentration and must be determined at the concentration of interest. The activity coefficient can also be obtained for non-volatile solutes, where the vapor pressure of the pure substance is negligible, by using the Gibbs–Duhem relation: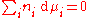
By measuring the change in vapor pressure (and hence chemical potential) of the solvent, the chemical potential of the solute can be deduced.
The standard state
for a dilute solution is also defined in terms of infinite-dilution behavior. Although the standard concentration co is taken to be 1 mol/L by convention, the standard state is a hypothetical solution of 1 mol/L in which the solute has its limiting infinite-dilution properties. This has the effect that all non-ideal behavior is described by the activity coefficient: the activity coefficient at 1 mol/L is not necessarily unity (and is frequently quite different from unity).
All the relations above can also be expressed in terms of molalities rather than concentrations, e.g.: , where
, where  for a volatile solutes; m
for a volatile solutes; mo = 1 mol/kg.
The standard chemical potential μmo, the activity coefficient γm and the Henry's law constant kH,m all have different numerical values when molalities are used in place of concentrations.
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
, Henry's law is one of the gas laws
Gas laws
The early gas laws were developed at the end of the 18th century, when scientists began to realize that relationships between the pressure, volume and temperature of a sample of gas could be obtained which would hold for all gases...
formulated by William Henry
William Henry (chemist)
William Henry was an English chemist.He was the son of Thomas Henry and was born in Manchester England. He developed what is known today as Henry's Law.-Life:...
in 1803. It states that:
- At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressurePartial pressureIn a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
of that gas in equilibrium with that liquid.
An equivalent way of stating the law is that the solubility
Solubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
of a gas in a liquid at a particular temperature is proportional to the pressure of that gas above the liquid. Henry's law has since been shown to apply for a wide range of dilute solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
s, not merely those of gases.
An everyday example of Henry's law is given by carbonated
Carbonation
Carbonation is the process of dissolving carbon dioxide in water. The process usually involves carbon dioxide under high pressure. When the pressure is reduced, the carbon dioxide is released from the solution as small bubbles, which cause the solution to "fizz." This effect is seen in carbonated...
soft drink
Soft drink
A soft drink is a non-alcoholic beverage that typically contains water , a sweetener, and a flavoring agent...
s. Before the bottle or can is opened, the gas above the drink is almost pure carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
at a pressure slightly higher than atmospheric pressure
Atmospheric pressure
Atmospheric pressure is the force per unit area exerted into a surface by the weight of air above that surface in the atmosphere of Earth . In most circumstances atmospheric pressure is closely approximated by the hydrostatic pressure caused by the weight of air above the measurement point...
. The drink itself contains dissolved carbon dioxide. When the bottle or can is opened, some of this gas escapes, giving the characteristic hiss (or "pop" in the case of a champagne bottle). Because the pressure above the liquid is now lower, some of the dissolved carbon dioxide comes out of solution as bubbles. If a glass of the drink is left in the open, the concentration of carbon dioxide in solution will come into equilibrium with the carbon dioxide in the air, and the drink will go "flat". Note that the pressure acting above the drink in the sealed must come from the partial pressure of carbon dioxide. If the gas is only air it would not produce the same effect even if the pressure value is the same.
A slightly more exotic example of Henry's law is in decompression
Decompression (diving)
Decompression in the context of diving derives from the reduction in ambient pressure experienced by the diver during the ascent at the end of a dive or hyperbaric exposure and refers to both the reduction in pressure and the process of allowing dissolved inert gases to be eliminated from the...
and decompression sickness
Decompression sickness
Decompression sickness describes a condition arising from dissolved gases coming out of solution into bubbles inside the body on depressurization...
of divers
Underwater diving
Underwater diving is the practice of going underwater, either with breathing apparatus or by breath-holding .Recreational diving is a popular activity...
.
Formula and the Henry's law constant
Henry's law can be put into mathematical terms (at constant temperature) as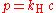
where p is the partial pressure
Partial pressure
In a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
of the solute
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
in the gas above the solution, c is the concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
of the solute and kH is a constant with the dimensions
Dimensional analysis
In physics and all science, dimensional analysis is a tool to find or check relations among physical quantities by using their dimensions. The dimension of a physical quantity is the combination of the basic physical dimensions which describe it; for example, speed has the dimension length per...
of pressure divided by concentration. The constant, known as the Henry's law constant, depends on the solute, the solvent and the temperature.
Some values for kH for gases dissolved in water at 298 K
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
include:
- oxygenOxygenOxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
(O2) : 769.2 LLitrepic|200px|right|thumb|One litre is equivalent to this cubeEach side is 10 cm1 litre water = 1 kilogram water The litre is a metric system unit of volume equal to 1 cubic decimetre , to 1,000 cubic centimetres , and to 1/1,000 cubic metre...
·atmAtmosphere (unit)The standard atmosphere is an international reference pressure defined as 101325 Pa and formerly used as unit of pressure. For practical purposes it has been replaced by the bar which is 105 Pa...
/molMole (unit)The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value... - carbon dioxideCarbon dioxideCarbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
(CO2) : 29.41 L·atm/mol - hydrogenHydrogenHydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
(H2) : 1282.1 L·atm/mol
There are other forms of Henry's Law, each of which defines the constant kH differently and requires different dimensional units. In particular, the "concentration" of the solute in solution may also be expressed as a mole fraction or as a molality.
Other forms of Henry's law
There are various other forms of Henry's Law which are discussed in the technical literature.| equation: | 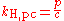 |
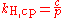 |
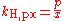 |
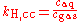 |
|---|---|---|---|---|
| units: |  |
 |
 |
dimensionless Dimensionless quantity In dimensional analysis, a dimensionless quantity or quantity of dimension one is a quantity without an associated physical dimension. It is thus a "pure" number, and as such always has a dimension of 1. Dimensionless quantities are widely used in mathematics, physics, engineering, economics, and... |
| O2 Oxygen Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition... |
769.23 | 1.3 | 4.259 | 3.181 |
| H2 Hydrogen Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly... |
1282.05 | 7.8 | 7.099 | 1.907 |
| CO2 Carbon dioxide Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom... |
29.41 | 3.4 | 0.163 | 0.8317 |
| N2 Nitrogen Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere... |
1639.34 | 6.1 | 9.077 | 1.492 |
| He Helium Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table... |
2702.7 | 3.7 | 14.97 | 9.051 |
| Ne Neon Neon is the chemical element that has the symbol Ne and an atomic number of 10. Although a very common element in the universe, it is rare on Earth. A colorless, inert noble gas under standard conditions, neon gives a distinct reddish-orange glow when used in either low-voltage neon glow lamps or... |
2222.22 | 4.5 | 12.30 | 1.101 |
| Ar Argon Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide... |
714.28 | 1.4 | 3.955 | 3.425 |
| CO Carbon monoxide Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal... |
1052.63 | 9.5 | 5.828 | 2.324 |
where:
- c = amount concentration of gas in solution (in mol/L)
- p = partial pressurePartial pressureIn a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
of gas above the solution (in atmAtmosphere (unit)The standard atmosphere is an international reference pressure defined as 101325 Pa and formerly used as unit of pressure. For practical purposes it has been replaced by the bar which is 105 Pa...
) - x = mole fraction of gas in solution (dimensionless)
As can be seen by comparing the equations in the above table, the Henry's law constant kH,pc is simply the inverse of the constant kH,cp. Since all kH may be referred to as Henry's law constants, readers of the technical literature must be quite careful to note which version of the Henry's Law equation is being used.
It should also be noted the Henry's Law is a limiting law that only applies for sufficiently dilute solutions. The range of concentrations in which it applies becomes narrower the more the system diverges from ideal behavior. Roughly speaking, that is the more chemically different the solute is from the solvent.
It also only applies simply for solutions where the solvent does not react chemically
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
with the gas being dissolved. A common example of a gas that does react with the solvent is carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, which forms carbonic acid
Carbonic acid
Carbonic acid is the inorganic compound with the formula H2CO3 . It is also a name sometimes given to solutions of carbon dioxide in water, because such solutions contain small amounts of H2CO3. Carbonic acid forms two kinds of salts, the carbonates and the bicarbonates...
(H2CO3) to a certain degree with water.
Temperature dependence of the Henry constant
When the temperature of a system changes, the Henry constant will also change. This is why some people prefer to name it Henry coefficient. There are multiple equations assessing the effect of temperature on the constant. These forms of the van 't Hoff equation are examples:
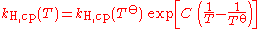
where
- kH for a given temperature is the Henry's Law constant (as defined in the first section of this article). Notice that the correct sign of C depends on whether kH,pc or kH,cp is used.
- T is the thermodynamic temperatureThermodynamic temperatureThermodynamic temperature is the absolute measure of temperature and is one of the principal parameters of thermodynamics. Thermodynamic temperature is an "absolute" scale because it is the measure of the fundamental property underlying temperature: its null or zero point, absolute zero, is the...
i.e. value in K], - T
orefers to the standard temperature (298 K).
This equation is only an approximation, and should be used only when no better, experimentally-derived formula is known for a given gas.
The following table lists some values for constant C (in kelvins) in the equation above:
| Gas | O2 Oxygen Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition... |
H2 Hydrogen Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly... |
CO2 | N2 Nitrogen Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere... |
He Helium Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table... |
Ne Neon Neon is the chemical element that has the symbol Ne and an atomic number of 10. Although a very common element in the universe, it is rare on Earth. A colorless, inert noble gas under standard conditions, neon gives a distinct reddish-orange glow when used in either low-voltage neon glow lamps or... |
Ar Argon Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide... |
CO Carbon monoxide Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal... |
| C(K) | 1700 | 500 | 2400 | 1300 | 230 | 490 | 1300 | 1300 |
Because solubility of permanent gases usually decreases with increasing temperature at around the room temperature, the partial pressure a given gas concentration has in liquid must increase. While heating water (saturated with nitrogen) from 25 °C to 95 °C the solubility will decrease to about 43% of its initial value. This can be verified when heating water in a pot: small bubbles evolve and rise, long before the water reaches boiling temperature. Similarly, carbon dioxide from a carbonated
Carbonation
Carbonation is the process of dissolving carbon dioxide in water. The process usually involves carbon dioxide under high pressure. When the pressure is reduced, the carbon dioxide is released from the solution as small bubbles, which cause the solution to "fizz." This effect is seen in carbonated...
drink escapes much faster when the drink is not cooled because the required partial pressure of CO2 to achieve the same solubility increases in higher temperatures. Partial pressure of CO2 in the gas phase in equilibrium with seawater doubles with every 16 K increase in temperature.
The constant C may be regarded as:
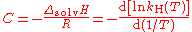
where
- ΔsolvH is the enthalpy of solution
- R is the gas constantGas constantThe gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
.
The solubility of gases does not always decrease with increasing temperature. For aqueous solutions, the Henry-law constant usually goes through a maximum (i.e., the solubility goes through a minimum). For most permanent gases, the minimum is below 120 °C. It is often observed that the smaller the gas molecule (and the lower the gas solubility in water), then the lower the temperature of the maximum of the Henry-law constant. Thus, the maximum is about 30 °C for helium, 92 to 93 °C for argon, nitrogen and oxygen, and 114 °C for xenon.
In geophysics
In geophysicsGeophysics
Geophysics is the physics of the Earth and its environment in space; also the study of the Earth using quantitative physical methods. The term geophysics sometimes refers only to the geological applications: Earth's shape; its gravitational and magnetic fields; its internal structure and...
, a version of Henry's law applies to the solubility of a noble gas
Noble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
in contact with silicate
Silicate
A silicate is a compound containing a silicon bearing anion. The great majority of silicates are oxides, but hexafluorosilicate and other anions are also included. This article focuses mainly on the Si-O anions. Silicates comprise the majority of the earth's crust, as well as the other...
melt. One equation used is
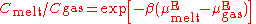
where:
- C = the number concentrations of the solute gas in the melt and gas phases
- β = 1/kBT, an inverse temperature scale: kB = the Boltzmann constant
- µE = the excess chemical potentialChemical potentialChemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
s of the solute gas in the two phases.
Comparison to Raoult's law
For a dilute solution, the concentration of the solute is approximately proportional to its mole fraction x, and Henry's law can be written as: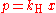
This can be compared with Raoult's law:
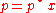
where p* is the vapor pressure of the pure component.
At first sight, Raoult's law appears to be a special case of Henry's law where kH = p*. This is true for pairs of closely related substances, such as benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
and toluene
Toluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
, which obey Raoult's law over the entire composition range: such mixtures are called "ideal mixtures".
The general case is that both laws are limit laws
Limit of a function
In mathematics, the limit of a function is a fundamental concept in calculus and analysis concerning the behavior of that function near a particular input....
, and they apply at opposite ends of the composition range. The vapor pressure of the component in large excess, such as the solvent for a dilute solution, is proportional to its mole fraction, and the constant of proportionality is the vapor pressure of the pure substance (Raoult's law). The vapor pressure of the solute is also proportional to the solute's mole fraction, but the constant of proportionality is different and must be determined experimentally (Henry's law). In mathematical terms:
- Raoult's law:
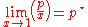
- Henry's law:
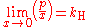
Raoult's law can also be related to non-gas solutes.
Standard chemical potential
Henry's law has been shown to apply to a wide range of solutes in the limit of "infinite dilution" (x→0), including non-volatile substances such as sucroseSucrose
Sucrose is the organic compound commonly known as table sugar and sometimes called saccharose. A white, odorless, crystalline powder with a sweet taste, it is best known for its role in human nutrition. The molecule is a disaccharide composed of glucose and fructose with the molecular formula...
or even sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
. In these cases, it is necessary to state the law in terms of chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
s. For a solute in an ideal dilute solution, the chemical potential depends on the concentration:
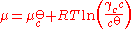 , where
, where 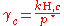 for a volatile solute; c
for a volatile solute; cFor non-ideal solutions, the activity coefficient
Activity coefficient
An activity coefficient is a factor used in thermodynamics to account for deviations from ideal behaviour in a mixture of chemical substances. In an ideal mixture, the interactions between each pair of chemical species are the same and, as a result, properties of the mixtures can be expressed...
γc depends on the concentration and must be determined at the concentration of interest. The activity coefficient can also be obtained for non-volatile solutes, where the vapor pressure of the pure substance is negligible, by using the Gibbs–Duhem relation:
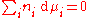
By measuring the change in vapor pressure (and hence chemical potential) of the solvent, the chemical potential of the solute can be deduced.
The standard state
Standard state
In chemistry, the standard state of a material is a reference point used to calculate its properties under different conditions. In principle, the choice of standard state is arbitrary, although the International Union of Pure and Applied Chemistry recommends a conventional set of standard states...
for a dilute solution is also defined in terms of infinite-dilution behavior. Although the standard concentration c
All the relations above can also be expressed in terms of molalities rather than concentrations, e.g.:
 , where
, where  for a volatile solutes; m
for a volatile solutes; mThe standard chemical potential μm
See also
- Bunsen solubility coefficientBunsen solubility coefficientThe Bunsen solubility coefficient or Bunsen absorption coefficient , named for Robert Bunsen, is one of a number of units used to describe the solubility of gases in liquids...
- Dalton's lawDalton's lawIn chemistry and physics, Dalton's law states that the total pressure exerted by a gaseous mixture is equal to the sum of the partial pressures of each individual component in a gas mixture...
- Partial pressurePartial pressureIn a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
- PervaporationPervaporationPervaporation is a membrane technical method for the separation of mixtures of liquids by partial vaporization through a non-porous or porous membrane.-Theory:...
External links
- www.henrys-law.org - Large compilation of Henry's law constants
- Ethanol solubility in EPDM - Solubility of chemicals in polymers using Henry's law
- New 'no air tanks' diving system, based on Henry's law - An article with flash presentation.
- http://dx.doi.org/10.1021/cr60242a003 - "The solubility of gases in liquids", R. Battino and H. Lawrence Clever, Chemical Reviews, 66, 4, 395-463 (1966).
- Henry's gas law calculator

