Halothane
Encyclopedia
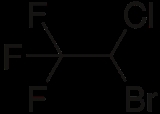
Halothane is an inhalational general anesthetic. Its IUPAC name is 2-bromo-2-chloro-1,1,1-trifluoroethane. It is the only inhalational anesthetic agent containing a bromine
atom; there are several other halogenated anesthesia agents which lack the bromine atom and do contain the fluorine and chlorine atoms present in halothane. It is colorless and pleasant-smelling, but unstable in light. It is packaged in dark-colored bottles and contains 0.01% thymol
as a stabilizing agent. Halothane is a core medicine in the World Health Organization
's "Essential Drugs List", which is a list of minimum medical needs for a basic health care system. Its use in developed countries, however, has been almost entirely superseded by newer inhalational anaesthetic agents.
of 0.74. Its blood gas coefficient of 2.4 makes it an agent with moderate induction and recovery time. It is not a good analgesic and its muscle relaxation effect is moderate.
; it is an alkyl halide. The structure has one stereocenter, so there are (R)- and (S)-optical isomers. Attempts to find anesthetics with less metabolism led to halogenated ether
s such as enflurane
and isoflurane
. The incidence of hepatic reactions with these agents is lower. The exact degree of hepatotoxic potential of enflurane is debated, although it is minimally metabolized. Isoflurane is essentially not metabolized and reports of associated liver injury are quite rare. Small amounts of trifluoroacetic acid
can be formed from both halothane and isoflurane metabolism and possibly accounts for cross sensitization of patients between these agents.
was first synthesized by C. W. Suckling
of Imperial Chemical Industries
(ICI) in 1951 and was first used clinically by M. Johnstone in Manchester
in 1956. Halothane became popular as a nonflammable general anasthetic replacing other volatile anesthetics such as diethyl ether
and cyclopropane
. Use of the anesthetic was phased out during the 1980s and 1990s as newer anesthetic agents became popular. Halothane retains some use in veterinary
surgery
and in the Third World
because of its lower cost.
Halothane was given to many millions of adult and pediatric patients worldwide from its introduction in 1956 through the 1980s. Its properties include cardiac depression at high levels, cardiac sensitization to catecholamine
s such as norepinephrine
, and potent bronchial relaxation. Its lack of airway irritation made it a common inhalation induction agent in pediatric anesthesia. Due to its cardiac depressive effect, it was contraindicated
in patients with cardiac failure. Halothane was also contraindicated in patients susceptible to cardiac arrhythmias, or in situations related to high catecholamine levels such as pheochromocytoma.
injury. This occurred in about 1 in 10,000 exposures. The resulting syndrome was referred to as halothane hepatitis
, and is thought to result from the metabolism of halothane to trifluoroacetic acid
via oxidative reactions in the liver. About 20% of inhaled halothane is metabolized by the liver and these products are excreted in the urine. The hepatitis syndrome had a mortality rate of 30% to 70%. Concern for hepatitis resulted in a dramatic reduction in the use of halothane for adults. It was replaced in the 1980s by enflurane
and isoflurane
. By the year 2005 the common volatile anesthetics in use were isoflurane
, sevoflurane
, and desflurane
. Since the risk of halothane hepatitis in children was substantially lower than in adults, halothane saw continued use in pediatrics in the 1990s. However, by the year 2000 sevoflurane
had largely replaced the use of halothane in children.
, which is reacted with hydrogen fluoride
in the presence of antimony trichloride
at 130 °C to form 2-chloro-1,1,1-trifluoroethane. This is then reacted with bromine
at 450 °C to produce halothane.

Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
atom; there are several other halogenated anesthesia agents which lack the bromine atom and do contain the fluorine and chlorine atoms present in halothane. It is colorless and pleasant-smelling, but unstable in light. It is packaged in dark-colored bottles and contains 0.01% thymol
Thymol
Thymol is a natural monoterpene phenol derivative of cymene, C10H14O, isomeric with carvacrol, found in oil of thyme, and extracted from Thymus vulgaris and various other kinds of plants as a white crystalline substance of a pleasant aromatic odor and strong antiseptic properties...
as a stabilizing agent. Halothane is a core medicine in the World Health Organization
World Health Organization
The World Health Organization is a specialized agency of the United Nations that acts as a coordinating authority on international public health. Established on 7 April 1948, with headquarters in Geneva, Switzerland, the agency inherited the mandate and resources of its predecessor, the Health...
's "Essential Drugs List", which is a list of minimum medical needs for a basic health care system. Its use in developed countries, however, has been almost entirely superseded by newer inhalational anaesthetic agents.
Anesthetic properties
It is a potent anesthetic with a minimum alveolar concentrationMinimum alveolar concentration
Minimum alveolar concentration or MAC is a concept used to compare the strengths, or potency, of anaesthetic vapours; in simple terms, it is defined as the concentration of the vapour in the lungs that is needed to prevent movement in 50% of subjects in response to surgical stimulus...
of 0.74. Its blood gas coefficient of 2.4 makes it an agent with moderate induction and recovery time. It is not a good analgesic and its muscle relaxation effect is moderate.
Related substances
Chemically, halothane is not an etherEther
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
; it is an alkyl halide. The structure has one stereocenter, so there are (R)- and (S)-optical isomers. Attempts to find anesthetics with less metabolism led to halogenated ether
Halogenated ether
A halogenated ether is a subcategory of a larger group of chemicals known as ethers. An ether is an organic chemical that contains an ether group — an oxygen atom connected to two alkyl groups...
s such as enflurane
Enflurane
Enflurane is a halogenated ether that was commonly used for inhalational anesthesia during the 1970s and 1980s. Developed by Ross Terrell in 1963, it was first used clinically in 1966....
and isoflurane
Isoflurane
Isoflurane is a halogenated ether used for inhalational anesthesia. Together with enflurane and halothane, it replaced the flammable ethers used in the pioneer days of surgery. Its name comes from being a structural isomer of enflurane, hence they have the same empirical formula...
. The incidence of hepatic reactions with these agents is lower. The exact degree of hepatotoxic potential of enflurane is debated, although it is minimally metabolized. Isoflurane is essentially not metabolized and reports of associated liver injury are quite rare. Small amounts of trifluoroacetic acid
Trifluoroacetic acid
Trifluoroacetic acid is the simplest stable perfluorinated carboxylic acid chemical compound, with the formula CF3CO2H. It is a strong carboxylic acid due to the influence of the electronegative trifluoromethyl group. TFA is almost 100,000-fold more acidic than acetic acid...
can be formed from both halothane and isoflurane metabolism and possibly accounts for cross sensitization of patients between these agents.
History
This halogenated hydrocarbonHaloalkane
The haloalkanes are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and...
was first synthesized by C. W. Suckling
Charles Suckling
Charles Walter Suckling, Commander of the Order of the British Empire is a British chemist who first synthesised halothane, a volatile inhalational anaesthetic in 1951, while working at the Imperial Chemical Industries Central Laboratory in Widnes.-Biography:He was born in Teddington, London,...
of Imperial Chemical Industries
Imperial Chemical Industries
Imperial Chemical Industries was a British chemical company, taken over by AkzoNobel, a Dutch conglomerate, one of the largest chemical producers in the world. In its heyday, ICI was the largest manufacturing company in the British Empire, and commonly regarded as a "bellwether of the British...
(ICI) in 1951 and was first used clinically by M. Johnstone in Manchester
Manchester
Manchester is a city and metropolitan borough in Greater Manchester, England. According to the Office for National Statistics, the 2010 mid-year population estimate for Manchester was 498,800. Manchester lies within one of the UK's largest metropolitan areas, the metropolitan county of Greater...
in 1956. Halothane became popular as a nonflammable general anasthetic replacing other volatile anesthetics such as diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
and cyclopropane
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
. Use of the anesthetic was phased out during the 1980s and 1990s as newer anesthetic agents became popular. Halothane retains some use in veterinary
Veterinary medicine
Veterinary Medicine is the branch of science that deals with the prevention, diagnosis and treatment of disease, disorder and injury in non-human animals...
surgery
Surgery
Surgery is an ancient medical specialty that uses operative manual and instrumental techniques on a patient to investigate and/or treat a pathological condition such as disease or injury, or to help improve bodily function or appearance.An act of performing surgery may be called a surgical...
and in the Third World
Third World
The term Third World arose during the Cold War to define countries that remained non-aligned with either capitalism and NATO , or communism and the Soviet Union...
because of its lower cost.
Halothane was given to many millions of adult and pediatric patients worldwide from its introduction in 1956 through the 1980s. Its properties include cardiac depression at high levels, cardiac sensitization to catecholamine
Catecholamine
Catecholamines are molecules that have a catechol nucleus consisting of benzene with two hydroxyl side groups and a side-chain amine. They include dopamine, as well as the "fight-or-flight" hormones adrenaline and noradrenaline released by the adrenal medulla of the adrenal glands in response to...
s such as norepinephrine
Norepinephrine
Norepinephrine is the US name for noradrenaline , a catecholamine with multiple roles including as a hormone and a neurotransmitter...
, and potent bronchial relaxation. Its lack of airway irritation made it a common inhalation induction agent in pediatric anesthesia. Due to its cardiac depressive effect, it was contraindicated
Contraindication
In medicine, a contraindication is a condition or factor that serves as a reason to withhold a certain medical treatment.Some contraindications are absolute, meaning that there are no reasonable circumstances for undertaking a course of action...
in patients with cardiac failure. Halothane was also contraindicated in patients susceptible to cardiac arrhythmias, or in situations related to high catecholamine levels such as pheochromocytoma.
Side effects
Repeated exposure to halothane in adults was noted in rare cases to result in severe liverLiver
The liver is a vital organ present in vertebrates and some other animals. It has a wide range of functions, including detoxification, protein synthesis, and production of biochemicals necessary for digestion...
injury. This occurred in about 1 in 10,000 exposures. The resulting syndrome was referred to as halothane hepatitis
Hepatitis
Hepatitis is a medical condition defined by the inflammation of the liver and characterized by the presence of inflammatory cells in the tissue of the organ. The name is from the Greek hepar , the root being hepat- , meaning liver, and suffix -itis, meaning "inflammation"...
, and is thought to result from the metabolism of halothane to trifluoroacetic acid
Trifluoroacetic acid
Trifluoroacetic acid is the simplest stable perfluorinated carboxylic acid chemical compound, with the formula CF3CO2H. It is a strong carboxylic acid due to the influence of the electronegative trifluoromethyl group. TFA is almost 100,000-fold more acidic than acetic acid...
via oxidative reactions in the liver. About 20% of inhaled halothane is metabolized by the liver and these products are excreted in the urine. The hepatitis syndrome had a mortality rate of 30% to 70%. Concern for hepatitis resulted in a dramatic reduction in the use of halothane for adults. It was replaced in the 1980s by enflurane
Enflurane
Enflurane is a halogenated ether that was commonly used for inhalational anesthesia during the 1970s and 1980s. Developed by Ross Terrell in 1963, it was first used clinically in 1966....
and isoflurane
Isoflurane
Isoflurane is a halogenated ether used for inhalational anesthesia. Together with enflurane and halothane, it replaced the flammable ethers used in the pioneer days of surgery. Its name comes from being a structural isomer of enflurane, hence they have the same empirical formula...
. By the year 2005 the common volatile anesthetics in use were isoflurane
Isoflurane
Isoflurane is a halogenated ether used for inhalational anesthesia. Together with enflurane and halothane, it replaced the flammable ethers used in the pioneer days of surgery. Its name comes from being a structural isomer of enflurane, hence they have the same empirical formula...
, sevoflurane
Sevoflurane
Sevoflurane , also called fluoromethyl hexafluoroisopropyl ether, is a sweet-smelling, nonflammable, highly fluorinated methyl isopropyl ether used for induction and maintenance of general anesthesia. Together with desflurane, it is replacing isoflurane and halothane in modern anesthesiology...
, and desflurane
Desflurane
Desflurane is a highly fluorinated methyl ethyl ether used for maintenance of general anesthesia. Like halothane, enflurane and isoflurane, it is a racemic mixture of and optical isomers...
. Since the risk of halothane hepatitis in children was substantially lower than in adults, halothane saw continued use in pediatrics in the 1990s. However, by the year 2000 sevoflurane
Sevoflurane
Sevoflurane , also called fluoromethyl hexafluoroisopropyl ether, is a sweet-smelling, nonflammable, highly fluorinated methyl isopropyl ether used for induction and maintenance of general anesthesia. Together with desflurane, it is replacing isoflurane and halothane in modern anesthesiology...
had largely replaced the use of halothane in children.
Physical properties
| Boiling point Boiling point The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid.... : |
50.2 °C | (at 101.325 kPa) |
| Density Density The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight... : |
1.868 g/cm³ | (at 20 °C) |
| Molecular Weight: | 197.4 u | |
| Vapor pressure Vapor pressure Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form... : |
244 mmHg | (at 20 °C) |
| 288 mmHg | (at 24 °C) | |
| MAC Minimum alveolar concentration Minimum alveolar concentration or MAC is a concept used to compare the strengths, or potency, of anaesthetic vapours; in simple terms, it is defined as the concentration of the vapour in the lungs that is needed to prevent movement in 50% of subjects in response to surgical stimulus... : |
0.75 | vol % |
| Blood:Gas Partition coefficient: | 2.5 | |
| Oil:Gas Partition coefficient: | 224 |
Synthesis
The commercial synthesis of halothane starts from trichloroethyleneTrichloroethylene
The chemical compound trichloroethylene is a chlorinated hydrocarbon commonly used as an industrial solvent. It is a clear non-flammable liquid with a sweet smell. It should not be confused with the similar 1,1,1-trichloroethane, which is commonly known as chlorothene.The IUPAC name is...
, which is reacted with hydrogen fluoride
Hydrogen fluoride
Hydrogen fluoride is a chemical compound with the formula HF. This colorless gas is the principal industrial source of fluorine, often in the aqueous form as hydrofluoric acid, and thus is the precursor to many important compounds including pharmaceuticals and polymers . HF is widely used in the...
in the presence of antimony trichloride
Antimony trichloride
Antimony trichloride is the chemical compound with the formula SbCl3. The soft colorless solid with a pungent odor was known to the alchemists as butter of antimony.-Preparation:...
at 130 °C to form 2-chloro-1,1,1-trifluoroethane. This is then reacted with bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
at 450 °C to produce halothane.

Further reading
- Atkinson, Rushman, Lee. A Synopsis of Anaesthesia. 1987.
- Eger, Eisenkraft, Weiskopf. The Pharmacology of Inhaled Anesthetics. 2003.

