
Exchange current density
Encyclopedia
In electrochemistry
, exchange current density is a parameter used in the Tafel equation
, Butler-Volmer equation and other expressions. The Tafel equation describes the dependence of current for an electrolytic process to overpotential
.
The exchange current density is that current in the absence of net electrolysis and at zero overpotential. The exchange current can be thought of as a background current to which the net current observed at various overpotentials is normalized. For a redox reaction written as a reduction at the equilibrium potential, electron transfer processes continue at electrode/solution interface in both directions. The cathodic current is balanced by the anodic current. This ongoing current in both directions is called the exchange current density. When the potential is set more negative than the formal potential, the cathodic current is greater than the anodic current. Written as a reduction, cathodic current is positive. The net current density is the difference between the cathodic and anodic current density.
Exchange current densities reflect intrinsic rates of electron transfer
between an analyte
and the electrode
. Such rates provide insights into the structure and bonding in the analyte and the electrode. For example, the exchange current densities for platinum
and mercury
electrodes for reduction of protons differ by a factor of 1010, indicative of the excellent catalytic properties of platinum. Owing to this difference, mercury is preferred electrode material at reducing (cathodic) potentials in aqueous solution.
oxides, species adsorbed species on the surface. The nature of the electroactive species (the analyte) in the solution also critically affects the exchange current densities, both the reduced and oxidized form.
Less important but still relevant are the environment of the solution including the solvent, nature of other electrolyte, and temperature. For the concentration dependence of the exchange current density, the following expression is given for a one-electron reaction: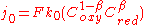
Electrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
, exchange current density is a parameter used in the Tafel equation
Tafel equation
The Tafel equation is an equation in electrochemical kinetics relating the rate of an electrochemical reaction to the overpotential. The Tafel equation was first deduced experimentally and was later shown to have a theoretical justification...
, Butler-Volmer equation and other expressions. The Tafel equation describes the dependence of current for an electrolytic process to overpotential
Overpotential
Overpotential is an electrochemical term which refers to the potential difference between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly related to a cell's voltage efficiency...
.
The exchange current density is that current in the absence of net electrolysis and at zero overpotential. The exchange current can be thought of as a background current to which the net current observed at various overpotentials is normalized. For a redox reaction written as a reduction at the equilibrium potential, electron transfer processes continue at electrode/solution interface in both directions. The cathodic current is balanced by the anodic current. This ongoing current in both directions is called the exchange current density. When the potential is set more negative than the formal potential, the cathodic current is greater than the anodic current. Written as a reduction, cathodic current is positive. The net current density is the difference between the cathodic and anodic current density.
Exchange current densities reflect intrinsic rates of electron transfer
Electron transfer
Electron transfer is the process by which an electron moves from an atom or a chemical species to another atom or chemical species...
between an analyte
Analyte
An analyte, or component , is a substance or chemical constituent that is of interest in an analytical procedure. Grammatically, it is important to note that experiments always seek to measure properties of analytes—and that analytes themselves can never be measured. For instance, one cannot...
and the electrode
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
. Such rates provide insights into the structure and bonding in the analyte and the electrode. For example, the exchange current densities for platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
and mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
electrodes for reduction of protons differ by a factor of 1010, indicative of the excellent catalytic properties of platinum. Owing to this difference, mercury is preferred electrode material at reducing (cathodic) potentials in aqueous solution.
Parameters affecting exchange current density
The exchange current density depends critically on the nature of the electrode, not only its structure, but also physical parameters such as surface roughness. Of course, factors that change the composition of the electrode including passivatingPassivation
Passivation is the process of making a material "passive", and thus less reactive with surrounding air, water, or other gases or liquids. The goal is to inhibit corrosion, whether for structural or cosmetic reasons. Passivation of metals is usually achieved by the deposition of a layer of oxide...
oxides, species adsorbed species on the surface. The nature of the electroactive species (the analyte) in the solution also critically affects the exchange current densities, both the reduced and oxidized form.
Less important but still relevant are the environment of the solution including the solvent, nature of other electrolyte, and temperature. For the concentration dependence of the exchange current density, the following expression is given for a one-electron reaction:
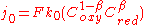
Example values
| Electrode material | Exchange current density -log10(A/cm2) |
|---|---|
| Palladium Palladium Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired... |
3.0 |
| Platinum Platinum Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal... |
3.1 |
| Rhodium Rhodium Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed... |
3.6 |
| Iridium Iridium Iridium is the chemical element with atomic number 77, and is represented by the symbol Ir. A very hard, brittle, silvery-white transition metal of the platinum family, iridium is the second-densest element and is the most corrosion-resistant metal, even at temperatures as high as 2000 °C... |
3.7 |
| Nickel Nickel Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile... |
5.2 |
| Gold Gold Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a... |
5.4 |
| Tungsten Tungsten Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as... |
5.9 |
| Niobium Niobium Niobium or columbium , is a chemical element with the symbol Nb and atomic number 41. It's a soft, grey, ductile transition metal, which is often found in the pyrochlore mineral, the main commercial source for niobium, and columbite... |
6.8 |
| Titanium Titanium Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color.... |
8.2 |
| Cadmium Cadmium Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low... |
10.8 |
| Manganese Manganese Manganese is a chemical element, designated by the symbol Mn. It has the atomic number 25. It is found as a free element in nature , and in many minerals... |
10.9 |
| Lead Lead Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed... |
12.0 |
| Mercury Mercury (element) Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum... |
12.3 |

