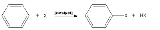
Electrophilic halogenation
Encyclopedia
In organic chemistry
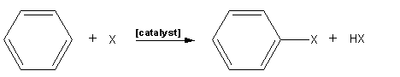
, an electrophilic aromatic halogenation is a type of electrophilic aromatic substitution
. This organic reaction
is typical of aromatic compounds and a very useful method for adding substituents to an aromatic system.
A few types of aromatic compounds, such as phenol
, will react without a catalyst, but for typical benzene derivatives with less reactive substrates, a Lewis acid
catalyst is required. Typical Lewis acid catalysts include AlCl3, FeCl3, FeBr3, and ZnCl2. These work by forming a highly electrophilic
complex
which attacks the benzene ring.
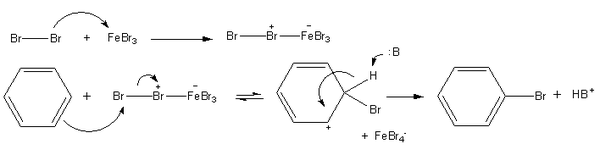
for chlorination of benzene is the same as bromination of benzene. Ferric bromide
and ferric chloride
become inactivated if they react with water, including moisture in the air. Therefore, they are generated by adding iron fillings to bromine or chlorine. Here is the mechanism of this reaction:
The mechanism for iodination is slightly different: iodine
(I2) is treated with an oxidizing agent such as nitric acid
to obtain the electrophilic iodine (2 I+). Unlike the other halogens, iodine does not serve as a base since it is positive. In one study the iodinization reagent is a mixture of iodine
and iodic acid
.
In another series of studies the powerful reagent obtained by using a mixture of iodine and potassium iodate
dissolved in concentrated sulfuric acid
was used. Here the iodinating agent is the tri-iodine cation I3+ and the base is HSO4-. In these studies both the kinetics of the reaction and the preparative conditions for the iodination of strongly deactivated compounds, such as benzoic acid
and 3-nitrobenzotrifluoride, were investigated.
Halogenation of aromatic compounds differs from the halogenation of alkenes, which do not require a Lewis Acid
catalyst. The formation of the arenium ion
results in the temporary loss of aromaticity
, which has a higher activation energy
compared to carbocation formation in alkenes. In other words, alkenes are more reactive and do not need to have the Br-Br or Cl-Cl bond weakened.
s, a catalyst is not necessary, for example in the bromination of p-cresol
:
However, if a catalyst is used with excess bromine, then a tribromide will be formed.
Halogenation of phenols is faster in polar solvents due to the dissociation of phenol, with phenoxide ions being more susceptible to electrophilic attack as they are more electron-rich.
Chlorination of toluene
with chlorine without catalyst requires a polar solvent as well such as acetic acid
. The ortho to para selectivity is low:
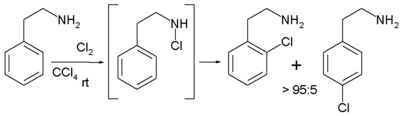
No reaction takes place when the solvent is replaced by tetrachloromethane. In contrast, when the reactant is 2-phenyl-ethylamine, it is possible to employ relatively apolar solvents with exclusive ortho- regioselectivity
due to the intermediate formation of a chloramine
making the subsequent reaction step intramolecular
.
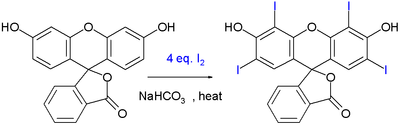
The food dye erythrosine
can be synthesized by iodination of another dye called fluorescein
:
This reaction is driven by sodium bicarbonate
.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, an electrophilic aromatic halogenation is a type of electrophilic aromatic substitution
Electrophilic aromatic substitution
Electrophilic aromatic substitution EAS is an organic reaction in which an atom, usually hydrogen, appended to an aromatic system is replaced by an electrophile...
. This organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
is typical of aromatic compounds and a very useful method for adding substituents to an aromatic system.
A few types of aromatic compounds, such as phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
, will react without a catalyst, but for typical benzene derivatives with less reactive substrates, a Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
catalyst is required. Typical Lewis acid catalysts include AlCl3, FeCl3, FeBr3, and ZnCl2. These work by forming a highly electrophilic
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
complex
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
which attacks the benzene ring.
Reaction mechanism
The reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
for chlorination of benzene is the same as bromination of benzene. Ferric bromide
Iron(III) bromide
Iron bromide is the chemical compound with the formula FeBr3. Also known as ferric bromide, this red-brown compound is used as a Lewis acid catalyst in the halogenation of aromatic compounds. It reacts with water to give acidic solutions....
and ferric chloride
Iron(III) chloride
Iron chloride, also called ferric chloride, is an industrial scale commodity chemical compound, with the formula FeCl3. The colour of iron chloride crystals depends on the viewing angle: by reflected light the crystals appear dark green, but by transmitted light they appear purple-red...
become inactivated if they react with water, including moisture in the air. Therefore, they are generated by adding iron fillings to bromine or chlorine. Here is the mechanism of this reaction:
The mechanism for iodination is slightly different: iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
(I2) is treated with an oxidizing agent such as nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
to obtain the electrophilic iodine (2 I+). Unlike the other halogens, iodine does not serve as a base since it is positive. In one study the iodinization reagent is a mixture of iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
and iodic acid
Iodic acid
Iodic acid, HIO3, can be obtained as a white solid. It dissolves in water very well, but it also exists in the pure state, as opposed to chloric acid or bromic acid. Iodic acid contains iodine in the oxidation state +5 and it is one of the most stable oxo-acids of the halogens in its pure state....
.
In another series of studies the powerful reagent obtained by using a mixture of iodine and potassium iodate
Potassium iodate
Potassium iodate is a chemical compound. It is ionic, made up of K+ ions and IO3- ions in a 1:1 ratio.-Chemical properties:Potassium iodate is an oxidizing agent and as such it can cause fires if in contact with combustible materials or reducing agents...
dissolved in concentrated sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
was used. Here the iodinating agent is the tri-iodine cation I3+ and the base is HSO4-. In these studies both the kinetics of the reaction and the preparative conditions for the iodination of strongly deactivated compounds, such as benzoic acid
Benzoic acid
Benzoic acid , C7H6O2 , is a colorless crystalline solid and the simplest aromatic carboxylic acid. The name derived from gum benzoin, which was for a long time the only source for benzoic acid. Its salts are used as a food preservative and benzoic acid is an important precursor for the synthesis...
and 3-nitrobenzotrifluoride, were investigated.
Halogenation of aromatic compounds differs from the halogenation of alkenes, which do not require a Lewis Acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
catalyst. The formation of the arenium ion
Arenium ion
An arenium ion in organic chemistry is a cyclohexadienyl cation that appears as a reactive intermediate in electrophilic aromatic substitution. For historic reasons this complex is also called a Wheland intermediate or a sigma complex or σ-complex.Two hydrogen atoms bonded to one carbon lie in a...
results in the temporary loss of aromaticity
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
, which has a higher activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
compared to carbocation formation in alkenes. In other words, alkenes are more reactive and do not need to have the Br-Br or Cl-Cl bond weakened.
Scope
If the ring contains a strongly activating substituent such as -OH, -OR or amineAmine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s, a catalyst is not necessary, for example in the bromination of p-cresol
Cresol
Cresols are organic compounds which are methylphenols. They are a widely occurring natural and manufactured group of aromatic organic compounds which are categorized as phenols . Depending on the temperature, cresols can be solid or liquid because they have melting points not far from room...
:
However, if a catalyst is used with excess bromine, then a tribromide will be formed.
Halogenation of phenols is faster in polar solvents due to the dissociation of phenol, with phenoxide ions being more susceptible to electrophilic attack as they are more electron-rich.
Chlorination of toluene
Toluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
with chlorine without catalyst requires a polar solvent as well such as acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
. The ortho to para selectivity is low:
No reaction takes place when the solvent is replaced by tetrachloromethane. In contrast, when the reactant is 2-phenyl-ethylamine, it is possible to employ relatively apolar solvents with exclusive ortho- regioselectivity
Regioselectivity
In chemistry, regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where...
due to the intermediate formation of a chloramine
Chloramine
Chloramines are derivatives of ammonia by substitution of one, two or three hydrogen atoms with chlorine atoms. Monochloramine is an inorganic compound with the formula NH2Cl. It is an unstable colourless liquid at its melting point of -66° temperature, but it is usually handled as a dilute...
making the subsequent reaction step intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
.
The food dye erythrosine
Erythrosine
Erythrosine, also known as Red No. 3, is an organoiodine compound, specifically a derivative of fluorone. It is cherry-pink synthetic, primarily used for food coloring. It is the disodium salt of 2,4,5,7-tetraiodofluorescein...
can be synthesized by iodination of another dye called fluorescein
Fluorescein
Fluorescein is a synthetic organic compound available as a dark orange/red powder soluble in water and alcohol. It is widely used as a fluorescent tracer for many applications....
:
This reaction is driven by sodium bicarbonate
Sodium bicarbonate
Sodium bicarbonate or sodium hydrogen carbonate is the chemical compound with the formula Na HCO3. Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda . The natural mineral form is...
.