
Dowd-Beckwith ring expansion reaction
Encyclopedia
The Dowd–Beckwith ring expansion reaction is an organic reaction
in which a cyclic β-keto
ester
is expanded by up to 4 carbons in a free radical
ring expansion reaction
through an α-alkylhalo substituent
. The radical initiator system is based on AIBN and tributyltin hydride
. The cyclic β-keto
ester
can be obtained through a Dieckmann condensation
. The original reaction consisted of a nucleophilic aliphatic substitution of the enolate of ethyl cyclohexanone-2-carboxylate with 1,4-diiodobutane and sodium hydride
followed by ring expansion to ethyl cyclodecanone-6-carboxylate. A side-reaction is organic reduction of the iodoalkane.
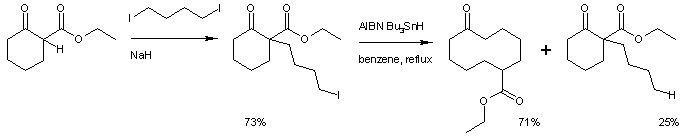
 The reaction mechanism
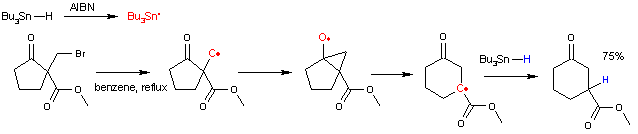
The reaction mechanism
involves a bicyclic intermediate. The reaction is initiated by thermal decomposition of AIBN. The resulting radicals
abstract hydrogen from tributyltin hydride
to a tributyltin radical which in turn abstracts the halogen
atom to form an alkyl radical. This radical attacks the carbonyl
group to an intermediate bicyclic ketyl
. This intermediate then rearranges
with ring expansion to a new carbon radical species which recombines with a proton radical from tributyltin hydride propagating the catalytic cycle
.
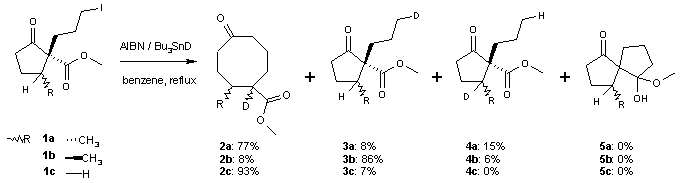 A side reaction accompanying this ring expansion is organic reduction of the halo alkane to a saturated alkyl group. One study shows that the success depends critically on the accessibility of the carbonyl group. Deuterium
A side reaction accompanying this ring expansion is organic reduction of the halo alkane to a saturated alkyl group. One study shows that the success depends critically on the accessibility of the carbonyl group. Deuterium
experiments also show the presence of a 1,5 hydride shift. The reaction of the alkyl radical with the ester carbonyl group is also a possibility but has an unfavorable activation energy
.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
in which a cyclic β-keto
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
is expanded by up to 4 carbons in a free radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
ring expansion reaction
Ring expansion reaction
In chemistry, ring expansion and ring contraction are chemical reactions that increase or decrease, respectively, the number of atoms in a ring of a cyclic compound.- Ring expansion reactions :Examples of ring expansion reactions are:...
through an α-alkylhalo substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
. The radical initiator system is based on AIBN and tributyltin hydride
Tributyltin hydride
Tributyltin hydride is an organotin compound with the formula 3SnH. It is a colorless liquid that is soluble in organic solvents. The compound is used as a source of hydrogen atoms in organic synthesis.-Synthesis and characterization:...
. The cyclic β-keto
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
can be obtained through a Dieckmann condensation
Dieckmann condensation
The Dieckmann condensation is the intramolecular chemical reaction of diesters with base to give β-ketoesters. It is named after the German chemist Walter Dieckmann . The equivalent intermolecular reaction is the Claisen condensation....
. The original reaction consisted of a nucleophilic aliphatic substitution of the enolate of ethyl cyclohexanone-2-carboxylate with 1,4-diiodobutane and sodium hydride
Sodium hydride
Sodium hydride is the chemical compound with the empirical formula NaH. It is primarily used as a strong base in organic synthesis. NaH is representative of the saline hydrides, meaning it is a salt-like hydride, composed of Na+ and H− ions, in contrast to the more molecular hydrides such as...
followed by ring expansion to ethyl cyclodecanone-6-carboxylate. A side-reaction is organic reduction of the iodoalkane.
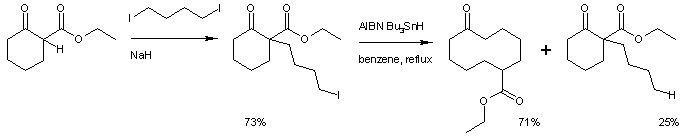
Reaction mechanism

Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
involves a bicyclic intermediate. The reaction is initiated by thermal decomposition of AIBN. The resulting radicals
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
abstract hydrogen from tributyltin hydride
Tributyltin hydride
Tributyltin hydride is an organotin compound with the formula 3SnH. It is a colorless liquid that is soluble in organic solvents. The compound is used as a source of hydrogen atoms in organic synthesis.-Synthesis and characterization:...
to a tributyltin radical which in turn abstracts the halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
atom to form an alkyl radical. This radical attacks the carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group to an intermediate bicyclic ketyl
Ketyl
A ketyl group in organic chemistry is an anion radical with the general structure C-O. in which an oxygen radical is bonded directly to carbon. This radical is very unstable and appears in chemical reactions as a reactive intermediate...
. This intermediate then rearranges
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
with ring expansion to a new carbon radical species which recombines with a proton radical from tributyltin hydride propagating the catalytic cycle
Catalytic cycle
A catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
.
Scope

Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
experiments also show the presence of a 1,5 hydride shift. The reaction of the alkyl radical with the ester carbonyl group is also a possibility but has an unfavorable activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
.

