
Crystal growth
Encyclopedia

Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. Crystal growth is a major stage of a crystallization process
Crystallization
Crystallization is the process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid...
, and consists in the addition of new atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s, ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s, or polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
strings into the characteristic arrangement of a crystalline Bravais lattice. The growth typically follows an initial stage of either homogeneous or heterogeneous (surface catalyzed) nucleation
Nucleation
Nucleation is the extremely localized budding of a distinct thermodynamic phase. Some examples of phases that may form by way of nucleation in liquids are gaseous bubbles, crystals or glassy regions. Creation of liquid droplets in saturated vapor is also characterized by nucleation...
, unless a "seed" crystal, purposely added to start the growth, was already present.
The action of crystal growth yields a crystalline solid whose atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s or molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s are typically close packed, with fixed positions in space
Space
Space is the boundless, three-dimensional extent in which objects and events occur and have relative position and direction. Physical space is often conceived in three linear dimensions, although modern physicists usually consider it, with time, to be part of a boundless four-dimensional continuum...
relative to each other.
The crystalline state of matter is characterized by a distinct structural rigidity
Structural rigidity
In discrete geometry and mechanics, structural rigidity is a combinatorial theory for predicting the flexibility of ensembles formed by rigid bodies connected by flexible linkages or hinges.-Definitions:...
and virtual resistance to deformation (i.e. changes of shape and/or volume). Most crystalline solids
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
have high values both of Young's modulus
Young's modulus
Young's modulus is a measure of the stiffness of an elastic material and is a quantity used to characterize materials. It is defined as the ratio of the uniaxial stress over the uniaxial strain in the range of stress in which Hooke's Law holds. In solid mechanics, the slope of the stress-strain...
and of the shear modulus of elasticity
Elasticity (physics)
In physics, elasticity is the physical property of a material that returns to its original shape after the stress that made it deform or distort is removed. The relative amount of deformation is called the strain....
. This contrasts with most liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
s or fluid
Fluid
In physics, a fluid is a substance that continually deforms under an applied shear stress. Fluids are a subset of the phases of matter and include liquids, gases, plasmas and, to some extent, plastic solids....
s, which have a low shear modulus, and typically exhibit the capacity for macroscopic viscous flow.
Introduction
Crystalline solids are typically formed by cooling and solidification from the molten (or liquidLiquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
) state. According to the Ehrenfest
Paul Ehrenfest
Paul Ehrenfest was an Austrian and Dutch physicist, who made major contributions to the field of statistical mechanics and its relations with quantum mechanics, including the theory of phase transition and the Ehrenfest theorem.- Biography :Paul Ehrenfest was born and grew up in Vienna in a Jewish...
classification of first-order phase transition
Phase transition
A phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....
s, there is a discontinuous change in volume (and thus a discontinuity in the slope or first derivative
Derivative
In calculus, a branch of mathematics, the derivative is a measure of how a function changes as its input changes. Loosely speaking, a derivative can be thought of as how much one quantity is changing in response to changes in some other quantity; for example, the derivative of the position of a...
with respect to temperature, dV/dT) at the melting point. Within this context, the crystal and melt are distinct phases with an interfacial discontinuity having a surface of tension
Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
with a positive surface energy
Surface energy
Surface energy quantifies the disruption of intermolecular bonds that occur when a surface is created. In the physics of solids, surfaces must be intrinsically less energetically favorable than the bulk of a material, otherwise there would be a driving force for surfaces to be created, removing...
. Thus, a metastable parent phase is always stable with respect to the nucleation
Nucleation
Nucleation is the extremely localized budding of a distinct thermodynamic phase. Some examples of phases that may form by way of nucleation in liquids are gaseous bubbles, crystals or glassy regions. Creation of liquid droplets in saturated vapor is also characterized by nucleation...
of small embryos or droplets from a daughter phase, provided it has a positive surface of tension. Such first-order transitions must proceed by the advancement of an interfacial region whose structure and properties vary discontinuously from the parent phase.
The process of nucleation and growth generally occurs in two different stages. In the first nucleation stage, a small nucleus containing the newly forming crystal is created. Nucleation occurs relatively slowly as the initial crystal components must impinge on each other in the correct orientation and placement for them to adhere and form the crystal. After crystal nucleation, the second stage of growth rapidly ensues. Crystal growth spreads outwards from the nucleating site. In this faster process, the elements which form the motif
Structural motif
In a chain-like biological molecule, such as a protein or nucleic acid, a structural motif is a supersecondary structure, which appears also in a variety of other molecules...
add to the growing crystal in a prearranged system, the crystal lattice, started in crystal nucleation. As first pointed out by Frank, perfect crystals would only grow exceedingly slowly. Real crystals grow comparatively rapidly because they contain dislocations (and other defects), which provide the necessary growth points, thus providing the necessary catalyst for structural transformation and long-range order formation.
Discontinuity
The conditions of a homogeneous environment are often approximated to but rarely ever realized. Crystal growth always involves some form of transport of matter or heat (or both). And homogeneous conditions for the transport process can only exist for spherical, cylindrical, or infinite plane surfaces. A polyhedral crystal cannot grow (remaining polyhedral) with uniform levels of supersaturation (or supercooling) over its faces. In general, the supersaturation is greatest at its corners. This refutes the assumption that the growth rate is a function of orientation and local supersaturation.Thus, the crystal face must grow as a whole. The growth rate of the entire face is determined by the driving force (level of supersaturation) at the point of emergence of the predominant point of growth (e.g. a dislocation, a foreign particle acting as catalyst, or crystal twin). The defect-free habit face can thus resist a finite level of supersaturation without any growth at all.
Gibbs himself was the first to point out that in the growth of a perfect crystal, the first derivative of the free energy with respect to mass becomes periodically undefinable — at each time that an additional layer on the crystal face is completed. There is discontinuity in the chemical potential at each such point.
In one sense, the crystal can then be in equilibrium with environments having a range of chemical potentials. In another sense, it is not in equilibrium. There are available states of lower free energy. But any free energy barrier must be passed by a fluctuation, or nucleation process, in order to access it. The fundamental thermodynamic effect of a screw dislocation is to eliminate this discontinuity in the chemical potential, by making it impossible to ever complete a single crystal face.
Nucleation
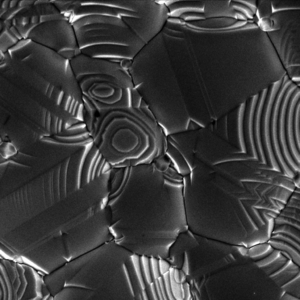
Heterogeneous nucleation can take place by several methods. Some of the most typical are small inclusions, or cuts, in the container the crystal is being grown on. This includes scratches on the sides and bottom of glassware. A common practice in crystal growing is to add a foreign substance, such as a string or a rock, to the solution, thereby providing nucleation sites for facilitating crystal growth and reducing the time to fully crystallize.
The number of nucleating sites can also be controlled in this manner. If a brand-new piece of glassware or a plastic container is used, crystals may not form because the container surface is too smooth to allow heterogeneous nucleation. On the other hand, a badly scratched container will result in many lines of small crystals. To achieve a moderate number of medium sized crystals, a container which has a few scratches works best. Likewise, adding small previously made crystals, or seed crystals, to a crystal growing project will provide nucleating sites to the solution. The addition of only one seed crystal should result in a larger single crystal.
Some important features during growth are the arrangement, the origin of growth, the interface form (important for the driving force), and the final size. When origin of growth is only in one direction for all the crystals, it can result in the material becoming very anisotropic (different properties in different directions). The interface form determines the additional free energy for each volume of crystal growth.
Lattice arrangement in metals often takes the structure of body centered cubic, face centered cubic, or hexagonal close packed. The final size of the crystal is important for mechanical properties of materials. (For example, in metals it is widely acknowledged that large crystals can stretch further due to the longer deformation path and thus lower internal stresses.).
Mechanisms of growth

- Non-uniform lateral growth. The surface advances by the lateral motion of steps which are one interplanar spacing in height (or some integral multiple thereof). An element of surface undergoes no change and does not advance normal to itself except during the passage of a step, and then it advances by the step height. It is useful to consider the step as the transition between two adjacent regions of a surface which are parallel to each other and thus identical in configuration — displaced from each other by an integral number of lattice planes. Note here the distinct possibility of a step in a diffuse surface, even though the step height would be much smaller than the thickness of the diffuse surface.
- Uniform normal growth. The surface advances normal to itself without the necessity of a stepwise growth mechanism. This means that in the presence of a sufficient thermodynamic driving force, every element of surface is capable of a continuous change contributing to the advancement of the interface. For a sharp or discontinuous surface, this continuous change may be more or less uniform over large areas each successive new layer. For a more diffuse surface, a continuous growth mechanism may require change over several successive layers simultaneously.
Non-uniform lateral growth is a geometrical motion of steps — as opposed to motion of the entire surface normal to itself. Alternatively, uniform normal growth is based on the time sequence of an element of surface. In this mode, there is no motion or change except when a step passes via a continual change. The prediction of which mechanism will be operative under any set of given conditions is fundamental to the understanding of crystal growth. Two criteria have been used to make this prediction:
- Whether or not the surface is diffuse. A diffuse surface is one in which the change from one phase to another is continuous, occurring over several atomic planes. This is in contrast to a sharp surface for which the major change in property (e.g. density or composition) is discontinuous, and is generally confined to a depth of one interplanar distance.
- Whether or not the surface is singular. A singular surface is one in which the surface tension as a function of orientation has a pointed minimum. Growth of singular surfaces is known to requires steps, whereas it is generally held that non-singular surfaces can continuously advance normal to themselves.
Driving force
Consider next the necessary requirements for the appearance of lateral growth. It is evident that the lateral growth mechanism will be found when any area in the surface can reach a metastable equilibrium in the presence of a driving force. It will then tend to remain in such an equilibrium configuration until the passage of a step. Afterward, the configuration will be identical except that each part of the step but will have advanced by the step height. If the surface cannot reach equilibrium in the presence of a driving force, then it will continue to advance without waiting for the lateral motion of steps.Thus, Cahn concluded that the distinguishing feature is the ability of the surface to reach an equilibrium state in the presence of the driving force. He also concluded that for every surface or interface in a crystalline medium, there exists a critical driving force, which, if exceeded, will enable the surface or interface to advance normal to itself, and, if not exceeded, will require the lateral growth mechanism.
Thus, for sufficiently large driving forces, the interface can move uniformly without the benefit of either a heterogeneous nucleation or screw dislocation mechanism. What constitutes a sufficiently large driving force depends upon the diffuseness of the interface, so that for extremely diffuse interfaces, this critical driving force will be so small that any measurable driving force will exceed it. Alternatively, for sharp interfaces, the critical driving force will be very large, and most growth will occur by the lateral step mechanism.
Note that in a typical solidification or crystallization
Crystallization
Crystallization is the process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid...
process, the thermodynamic driving force is dictated by the degree of supercooling
Supercooling
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid....
.
Morphology
It may be instructional to note that whisker
Monocrystalline whisker
A monocrystalline whisker is a filament of material that is structured as a single, defect-free crystal. Typical whisker materials are graphite, alumina, iron, or silicon. Single-crystal whiskers of these materials are noted for having very high tensile strength...
growth provides the link between the mechanical phenomenon of high strength in whiskers and the various growth mechanisms which are responsible for their fibrous morphologies. (Prior to the discovery of carbon nanotubes, single-crystal whiskers
Monocrystalline whisker
A monocrystalline whisker is a filament of material that is structured as a single, defect-free crystal. Typical whisker materials are graphite, alumina, iron, or silicon. Single-crystal whiskers of these materials are noted for having very high tensile strength...
had the highest tensile strength of any materials known). Some mechanisms produce defect-free whiskers, while others may have single screw dislocations along the main axis of growth — producing high strength whiskers.
The mechanism behind whisker growth is not well understood, but seems to be encouraged by compressive mechanical stress
Stress (physics)
In continuum mechanics, stress is a measure of the internal forces acting within a deformable body. Quantitatively, it is a measure of the average force per unit area of a surface within the body on which internal forces act. These internal forces are a reaction to external forces applied on the body...
es including mechanically induced stresses, stresses induced by diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
of different elements, and thermally induced stresses. Metal whiskers differ from metallic dendrite
Dendrite (crystal)
A crystal dendrite is a crystal that develops with a typical multi-branching tree-like form. Dendritic crystal growth is very common and illustrated by snowflake formation and frost patterns on a window. Dendritic crystallization forms a natural fractal pattern...
s in several respects. Dendrites are fern
Fern
A fern is any one of a group of about 12,000 species of plants belonging to the botanical group known as Pteridophyta. Unlike mosses, they have xylem and phloem . They have stems, leaves, and roots like other vascular plants...
-shaped like the branches of a tree, and grow across the surface of the metal. In contrast, whiskers are fibrous and project at a right angle to the surface of growth, or substrate.
Diffusion-control


In the subsequent tip-thickening process, there should be a corresponding instability of shape. Minor bumps or "bulges" should be exaggerated — and develop into rapidly growing side branches. In such an unstable (or metastable) situation, minor degrees of anisotropy should be sufficient to determine directions of significant branching and growth. The most appealing aspect of this argument, of course, is that it yields the primary morphological features of dendritic
Dendrite (crystal)
A crystal dendrite is a crystal that develops with a typical multi-branching tree-like form. Dendritic crystal growth is very common and illustrated by snowflake formation and frost patterns on a window. Dendritic crystallization forms a natural fractal pattern...
growth.
See also
- Cloud condensation nucleiCloud condensation nucleiCloud condensation nuclei or CCNs are small particles typically 0.2 µm, or 1/100 th the size of a cloud droplet ) about which cloud droplets coalesce. Water requires a non-gaseous surface to make the transition from a vapour to a liquid. In the atmosphere, this surface presents itself as tiny...
- Crystal structureCrystal structureIn mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
- CrystallizationCrystallizationCrystallization is the process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid...
- Czochralski processCzochralski processThe Czochralski process is a method of crystal growth used to obtain single crystals of semiconductors , metals , salts, and synthetic gemstones...
- Dendrite (crystal)Dendrite (crystal)A crystal dendrite is a crystal that develops with a typical multi-branching tree-like form. Dendritic crystal growth is very common and illustrated by snowflake formation and frost patterns on a window. Dendritic crystallization forms a natural fractal pattern...
- Fractional crystallizationFractional crystallization (chemistry)In chemistry, fractional crystallization is a method of refining substances based on differences in solubility. If a mixture of two or more substances in solution is allowed to crystallize, for example by allowing the temperature of the solution to decrease, the precipitate will contain more of...
- Phase separation
- Ice nucleusIce nucleusAn ice nucleus is a particle which acts as the nucleus for the formation of an ice crystal in the atmosphere.The presence of ice nuclei increase the temperature that ice will form in the atmosphere from around −42°C to about −10°C...
- Laser-heated pedestal growthLaser-heated pedestal growthLaser-heated pedestal growth is a crystal growth technique. The technique can be viewed as a miniature floating zone, where the heat source is replaced by a powerful CO2 or YAG laser...
- Manganese noduleManganese nodulePolymetallic nodules, also called manganese nodules, are rock concretions on the sea bottom formed of concentric layers of iron and manganese hydroxides around a core. The core may be microscopically small and is sometimes completely transformed into manganese minerals by crystallization...
- Micro-pulling-downMicro-pulling-down-Basics:The micro-pulling-down method is a crystal growth technique based on continuous transport of the melted substance through micro-channel made in a crucible bottom. Continuous solidification of the melt is progressed on a liquid/solid interface positioned under the crucible...
- Monocrystalline whiskerMonocrystalline whiskerA monocrystalline whisker is a filament of material that is structured as a single, defect-free crystal. Typical whisker materials are graphite, alumina, iron, or silicon. Single-crystal whiskers of these materials are noted for having very high tensile strength...
- ProtocrystallineProtocrystallineA protocrystalline phase is a distinct phase occurring during crystal growth which evolves into a microcrystalline form. The term is typically associated with silicon films in optical applications such as solar cells.-Silicon solar cells:...
- Recrystallization (chemistry)Recrystallization (chemistry)-Chemistry:In chemistry, recrystallization is a procedure for purifying compounds. The most typical situation is that a desired "compound A" is contaminated by a small amount of "impurity B". There are various methods of purification that may be attempted , which includes recrystallization...
- Seed crystalSeed crystalA seed crystal is a small piece of single crystal/polycrystal material from which a large crystal of the same material typically is to be grown...
- Single crystalSingle crystalA single crystal or monocrystalline solid is a material in which the crystal lattice of the entire sample is continuous and unbroken to the edges of the sample, with no grain boundaries...

