Coronin
Encyclopedia
Coronin is an actin
binding protein which also interacts with microtubule
s and in some cell types is associated with phagocytosis. Coronin proteins are expressed in a large number of eukaryotic organisms from yeast to man.
, which was later shown to bind actin in vitro. This actin binding protein was named coronin after its strong immunolocalisation in the actin rich crown like extension of the cell cortex in D. discoidium. Initially this protein was admitted into club of actin binding proteins with least enthusiasm, as the primary structure did not match any other ABPs. But later on, the protein was identified in many eukaryotic cells and Dictyostelium discoideum was found to be impaired in cytokinesis, and many actin mediated processes like endocytosis, cell motility etc.
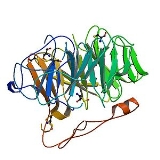
 Coronin belongs to WD-repeat containing proteins which form a beta propeller tertiary structure. The crystal structure of Coronin 1A (see figure to the right) containing major part of the protein was solved in 2006.
Coronin belongs to WD-repeat containing proteins which form a beta propeller tertiary structure. The crystal structure of Coronin 1A (see figure to the right) containing major part of the protein was solved in 2006.
The WD-repeat
is a structural motif
comprising approximately 40 amino acids usually ending with the amino acid sequence tryptophan
(W) – aspartic acid
(D) and hence the name WD.
WD-40-domain –repeat proteins are defined by the presence of at least four WD repeats located centrally in the protein. These domains were discovered in 1986 and are characterized by a partial conserved domain of 40-60 amino acids, starting with GH dipeptide 11-24 residue away from the N-terminus and ending with a tryptophane-aspartic acid (WD) dipeptide at the C-terminus. The WD domain has no ntrinsic catalytic activity and is thought to serve as a stable platform for simultaneous interaction. WD repeat proteins have diverse cellular functions. They play central role in physiological processes like signal transduction, transcriptional regulation, cytoskeleton remodeling, regulation of vesicle trafficking etc.
Coronin homologues both in vertebrates and invertebrates forms a subfamily among WD repeat proteins. Coronin contains 3-5 WD clustered repeats forming the central core domain. Apart from core domain, almost all coronins have a short conserved N-terminal motif and coiled coil motif of 50aa at C-terminus. The N-terminal region contains 12 basic aa which can be taken as signature as it is present in only coronin proteins. A recent study shows that these basic residues are involved in actin binding. Furthermore, each coronin contains a unique divergent region between the WD domain and C-terminal coiled coil region. The number of amino acids in this region varies greatly. The unique region has of dictyostelium has 22aa whereas mammalian coronins contains about 50 aa. The coronin like proteins from budding yeast Crn1 and one of the coronins in C. elegans has a much longer unique region i.e. 194 vs 144aa. The unique region of yeast coronin shows homologies with microtubule binding domains of the MAPs and yeast coronin binds both actin and microtubule and serve as bridge between them.
A second region of variability exists in the fourth β-strand of the third WD repeats.
Coronin homologue regulate actin cytoskeleton
and are involved in vesicular trafficking.
Seven different isoforms of Coronin have been reported in mammals. The most well studied isoforms are Coronin 1 (Coronin 1A) and Coronin 1B. Coronin 1 exerted an inhibitory effect on cellular steady-state F-actin formation via an Arp2/3-dependent mechanism. Whereas Coronin 1 was required for chemokine-mediated migration, it was dispensable for T cell antigen receptor functions in T cells. Coronin 1B is required for efficient cell protrusion and migration. Recent study demonstrates that Coronin 1B inhibits the Arp2/3 complex activity by replacing it at the branched actin structure. Mammalian Coronin-7 does not interact with actin nor does it execute any actin mediated processes, but rather participates in Golgi trafficking.
Although Coronin is present almost all eukaryotic organisms and have different functions, but everywhere these proteins have been shown to bind F-actin and localize in the dynamic F-actin rich area of cells. Recent study shows that Coronin prefers ATP/ADP-Pi containing F-actin over ADP containing F-actin, which might explain their unique cellular localization.
N-terminus signature region is reduced to 5aa and appears in front of each WD-repeat core domain (e.g., CRN7, POD-1)
Actin
Actin is a globular, roughly 42-kDa moonlighting protein found in all eukaryotic cells where it may be present at concentrations of over 100 μM. It is also one of the most highly-conserved proteins, differing by no more than 20% in species as diverse as algae and humans...
binding protein which also interacts with microtubule
Microtubule
Microtubules are a component of the cytoskeleton. These rope-like polymers of tubulin can grow as long as 25 micrometers and are highly dynamic. The outer diameter of microtubule is about 25 nm. Microtubules are important for maintaining cell structure, providing platforms for intracellular...
s and in some cell types is associated with phagocytosis. Coronin proteins are expressed in a large number of eukaryotic organisms from yeast to man.
Discovery
Eugenio L. de Hostos et al. (1991) isolated a 55 kDa protein from actinomyosin complex of Dictyostelium discoideumDictyostelium discoideum
Dictyostelium discoideum is a species of soil-living amoeba belonging to the phylum Mycetozoa. D. discoideum, commonly referred to as slime mold, is a eukaryote that transitions from a collection of unicellular amoebae into a multicellular slug and then into a fruiting body within its lifetime. D...
, which was later shown to bind actin in vitro. This actin binding protein was named coronin after its strong immunolocalisation in the actin rich crown like extension of the cell cortex in D. discoidium. Initially this protein was admitted into club of actin binding proteins with least enthusiasm, as the primary structure did not match any other ABPs. But later on, the protein was identified in many eukaryotic cells and Dictyostelium discoideum was found to be impaired in cytokinesis, and many actin mediated processes like endocytosis, cell motility etc.
Structure

The WD-repeat
WD40 repeat
The WD40 repeat is a short structural motif of approximately 40 amino acids , often terminating in a tryptophan-aspartic acid dipeptide...
is a structural motif
Structural motif
In a chain-like biological molecule, such as a protein or nucleic acid, a structural motif is a supersecondary structure, which appears also in a variety of other molecules...
comprising approximately 40 amino acids usually ending with the amino acid sequence tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
(W) – aspartic acid
Aspartic acid
Aspartic acid is an α-amino acid with the chemical formula HOOCCHCH2COOH. The carboxylate anion, salt, or ester of aspartic acid is known as aspartate. The L-isomer of aspartate is one of the 20 proteinogenic amino acids, i.e., the building blocks of proteins...
(D) and hence the name WD.
WD-40-domain –repeat proteins are defined by the presence of at least four WD repeats located centrally in the protein. These domains were discovered in 1986 and are characterized by a partial conserved domain of 40-60 amino acids, starting with GH dipeptide 11-24 residue away from the N-terminus and ending with a tryptophane-aspartic acid (WD) dipeptide at the C-terminus. The WD domain has no ntrinsic catalytic activity and is thought to serve as a stable platform for simultaneous interaction. WD repeat proteins have diverse cellular functions. They play central role in physiological processes like signal transduction, transcriptional regulation, cytoskeleton remodeling, regulation of vesicle trafficking etc.
Coronin homologues both in vertebrates and invertebrates forms a subfamily among WD repeat proteins. Coronin contains 3-5 WD clustered repeats forming the central core domain. Apart from core domain, almost all coronins have a short conserved N-terminal motif and coiled coil motif of 50aa at C-terminus. The N-terminal region contains 12 basic aa which can be taken as signature as it is present in only coronin proteins. A recent study shows that these basic residues are involved in actin binding. Furthermore, each coronin contains a unique divergent region between the WD domain and C-terminal coiled coil region. The number of amino acids in this region varies greatly. The unique region has of dictyostelium has 22aa whereas mammalian coronins contains about 50 aa. The coronin like proteins from budding yeast Crn1 and one of the coronins in C. elegans has a much longer unique region i.e. 194 vs 144aa. The unique region of yeast coronin shows homologies with microtubule binding domains of the MAPs and yeast coronin binds both actin and microtubule and serve as bridge between them.
A second region of variability exists in the fourth β-strand of the third WD repeats.
Function
Yeast Coronin Crn1 and Drosophila Dpod1 were found crosslink actin and microtubule cytoskeleton. C.elegans POD-1 and DrosophilaDrosophila
Drosophila is a genus of small flies, belonging to the family Drosophilidae, whose members are often called "fruit flies" or more appropriately pomace flies, vinegar flies, or wine flies, a reference to the characteristic of many species to linger around overripe or rotting fruit...
Coronin homologue regulate actin cytoskeleton
Cytoskeleton
The cytoskeleton is a cellular "scaffolding" or "skeleton" contained within a cell's cytoplasm and is made out of protein. The cytoskeleton is present in all cells; it was once thought to be unique to eukaryotes, but recent research has identified the prokaryotic cytoskeleton...
and are involved in vesicular trafficking.
Seven different isoforms of Coronin have been reported in mammals. The most well studied isoforms are Coronin 1 (Coronin 1A) and Coronin 1B. Coronin 1 exerted an inhibitory effect on cellular steady-state F-actin formation via an Arp2/3-dependent mechanism. Whereas Coronin 1 was required for chemokine-mediated migration, it was dispensable for T cell antigen receptor functions in T cells. Coronin 1B is required for efficient cell protrusion and migration. Recent study demonstrates that Coronin 1B inhibits the Arp2/3 complex activity by replacing it at the branched actin structure. Mammalian Coronin-7 does not interact with actin nor does it execute any actin mediated processes, but rather participates in Golgi trafficking.
Although Coronin is present almost all eukaryotic organisms and have different functions, but everywhere these proteins have been shown to bind F-actin and localize in the dynamic F-actin rich area of cells. Recent study shows that Coronin prefers ATP/ADP-Pi containing F-actin over ADP containing F-actin, which might explain their unique cellular localization.
Classification
- Short conventional coronins. Contain 450-650 amino acids with C-terminus coiled coil region of 30-40 aa that mediates homophilic dimerization and or olimerization of coronins (e.g., CRN1, CRN2).
- Long coronins. two core domains with no C-terminal coiled coil region
N-terminus signature region is reduced to 5aa and appears in front of each WD-repeat core domain (e.g., CRN7, POD-1)
Family members
Human proteins which are members of the coronin family include:- CORO1ACORO1ACoronin-1A is a protein that in humans is encoded by the CORO1A gene. It has been implicated in both T-cell mediated immunity and mitochondrial apoptosis. In a recent genome-wide longevity study, its expression levels were found to be negatively associated both with age at the time of blood sample...
- CORO1BCORO1BCoronin, actin binding protein, 1B also known as CORO1B is a protein which in humans is encoded by the CORO1B gene. Members of the coronin family, such as CORO1B, are WD repeat-containing actin-binding proteins that regulate cell motility....
- CORO1CCORO1CCoronin-1C is a protein that in humans is encoded by the CORO1C gene.-Further reading:...
- CORO2A
- CORO2B
- CORO6
- CORO7

