_chloride.gif)
Cerium(III) chloride
Encyclopedia
Cerium chloride (CeCl3), also known as cerous chloride or cerium trichloride, is a compound of cerium
and chlorine
. It is a white hygroscopic solid; It rapidly absorbs water on exposure to moist air to form a hydrate
which appears to be of variable composition, though the heptahydrate CeCl3·7 H2O is known. It is highly soluble in water, and (when anhydrous) it is soluble in ethanol
and acetone
.
. A useful form of anhydrous CeCl3 can be prepared if care is taken to heat the heptahydrate gradually to 140 °C (284 °F) over many hours under vacuum. This may or may not contain a little CeOCl from hydrolysis
), but it is suitable for use with organolithium
and Grignard reagents. Pure anhydrous
CeCl3 can be made by dehydration of the hydrate either by slowly heating to 400 °C (752 °F) with 4-6 equivalents of ammonium chloride
under high vacuum, or by heating with an excess of thionyl chloride
for three hours. The anhydrous
halide
may alternatively be prepared from cerium
metal and hydrogen chloride
. It is usually purified by high temperature sublimation under high vacuum.
salt
s, such as the Lewis acid
, cerium(III) trifluoromethanesulfonate, used for Friedel-Crafts acylations. It is also used itself as a Lewis acid
, for example as a catalyst in Friedel-Crafts alkylation
reactions.
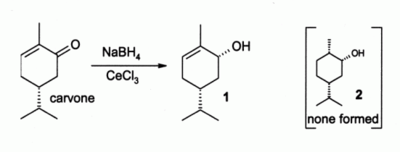
Luche reduction
of alpha, beta-unsaturated carbonyl compounds has become a popular method in organic synthesis
, where CeCl3.7H2O is used in conjunction with sodium borohydride
. For example carvone
gives only the allylic alcohol
1 and none of the saturated
alcohol
2. Without CeCl3, a mixture of 1 and 2 is formed.
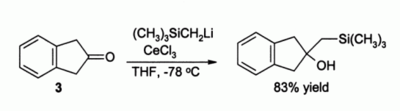
 Another important use in organic synthesis
Another important use in organic synthesis
is for alkylation
of ketone
s which would otherwise form enolates if simple organolithium reagent
s were to be used. For example, compound 3 would be expected to simply form an enolate without CeCl3 being present, but in the presence of CeCl3 smooth alkylation
occurs:
 It is reported that organolithium
It is reported that organolithium
s work more effectively in this reaction than do Grignard reagents.
Cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. It is a soft, silvery, ductile metal which easily oxidizes in air. Cerium was named after the dwarf planet . Cerium is the most abundant of the rare earth elements, making up about 0.0046% of the Earth's crust by weight...
and chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
. It is a white hygroscopic solid; It rapidly absorbs water on exposure to moist air to form a hydrate
Hydrate
Hydrate is a term used in inorganic chemistry and organic chemistry to indicate that a substance contains water. The chemical state of the water varies widely between hydrates, some of which were so labeled before their chemical structure was understood....
which appears to be of variable composition, though the heptahydrate CeCl3·7 H2O is known. It is highly soluble in water, and (when anhydrous) it is soluble in ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
and acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
.
Preparation of anhydrous CeCl3
Simple rapid heating of the hydrate alone may cause small amounts of hydrolysisHydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
. A useful form of anhydrous CeCl3 can be prepared if care is taken to heat the heptahydrate gradually to 140 °C (284 °F) over many hours under vacuum. This may or may not contain a little CeOCl from hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
), but it is suitable for use with organolithium
Organolithium reagent
An organolithium reagent is an organometallic compound with a direct bond between a carbon and a lithium atom. As the electropositive nature of lithium puts most of the charge density of the bond on the carbon atom, effectively creating a carbanion, organolithium compounds are extremely powerful...
and Grignard reagents. Pure anhydrous
Anhydrous
As a general term, a substance is said to be anhydrous if it contains no water. The way of achieving the anhydrous form differs from one substance to another...
CeCl3 can be made by dehydration of the hydrate either by slowly heating to 400 °C (752 °F) with 4-6 equivalents of ammonium chloride
Ammonium chloride
Ammonium chloride NH4Cl is an inorganic compound with the formula NH4Cl. It is a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. Sal ammoniac is a name of natural, mineralogical form of ammonium chloride...
under high vacuum, or by heating with an excess of thionyl chloride
Thionyl chloride
Thionyl chloride is an inorganic compound with the formula SOCl2. It is a reactive chemical reagent used in chlorination reactions. It is a colorless, distillable liquid at room temperature and pressure that decomposes above 140 °C. Thionyl chloride is sometimes confused with sulfuryl...
for three hours. The anhydrous
Anhydrous
As a general term, a substance is said to be anhydrous if it contains no water. The way of achieving the anhydrous form differs from one substance to another...
halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
may alternatively be prepared from cerium
Cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. It is a soft, silvery, ductile metal which easily oxidizes in air. Cerium was named after the dwarf planet . Cerium is the most abundant of the rare earth elements, making up about 0.0046% of the Earth's crust by weight...
metal and hydrogen chloride
Hydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
. It is usually purified by high temperature sublimation under high vacuum.
Uses
Cerium(III) chloride can be used as a starting point for the preparation of other ceriumCerium
Cerium is a chemical element with the symbol Ce and atomic number 58. It is a soft, silvery, ductile metal which easily oxidizes in air. Cerium was named after the dwarf planet . Cerium is the most abundant of the rare earth elements, making up about 0.0046% of the Earth's crust by weight...
salt
Salt
In chemistry, salts are ionic compounds that result from the neutralization reaction of an acid and a base. They are composed of cations and anions so that the product is electrically neutral...
s, such as the Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
, cerium(III) trifluoromethanesulfonate, used for Friedel-Crafts acylations. It is also used itself as a Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
, for example as a catalyst in Friedel-Crafts alkylation
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
reactions.
Luche reduction
Luche reduction
Luche reduction is the selective organic reduction of ketones to alcohols with lanthanoid chlorides such as cerium chloride and sodium borohydride. The Luche reduction can be conducted chemoselectively toward ketone in the presence of aldehyde or toward α,β-unsaturated ketone in the presence of...
of alpha, beta-unsaturated carbonyl compounds has become a popular method in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
, where CeCl3.7H2O is used in conjunction with sodium borohydride
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
. For example carvone
Carvone
Carvone is a member of a family of chemicals called terpenoids. Carvone is found naturally in many essential oils, but is most abundant in the oils from seeds of caraway and dill.-Stereoisomerism and odor:...
gives only the allylic alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
1 and none of the saturated
Saturation (chemistry)
In chemistry, saturation has six different meanings, all based on reaching a maximum capacity...
alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
2. Without CeCl3, a mixture of 1 and 2 is formed.

Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
is for alkylation
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
of ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s which would otherwise form enolates if simple organolithium reagent
Organolithium reagent
An organolithium reagent is an organometallic compound with a direct bond between a carbon and a lithium atom. As the electropositive nature of lithium puts most of the charge density of the bond on the carbon atom, effectively creating a carbanion, organolithium compounds are extremely powerful...
s were to be used. For example, compound 3 would be expected to simply form an enolate without CeCl3 being present, but in the presence of CeCl3 smooth alkylation
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
occurs:

Organolithium reagent
An organolithium reagent is an organometallic compound with a direct bond between a carbon and a lithium atom. As the electropositive nature of lithium puts most of the charge density of the bond on the carbon atom, effectively creating a carbanion, organolithium compounds are extremely powerful...
s work more effectively in this reaction than do Grignard reagents.
Further reading
- CRC Handbook of Chemistry and Physics (58th edition), CRC Press, West Palm Beach, Florida, 1977.

