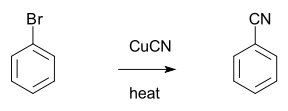
Rosenmund-von Braun synthesis
Encyclopedia
The Rosenmund-von Braun synthesis is an organic reaction
in which an aryl
halide
reacts with cuprous cyanide to an aryl
nitrile
..
The reaction was named after Karl Wilhelm Rosenmund
who together with his Ph.D. student Erich Struck discovered in 1914 that aryl
halide
reacts with alcohol water solution of potassium cyanide
and catalytic amounts of cuprous cyanide at 200°C. The reaction yields the carboxylic acid
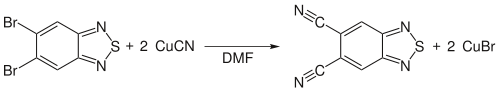
, not the nitrile, but Rosenmund speculated that the intermediate should be the nitrile. Independently Alfred Pongratz and Julius von Braun improved the reaction by changing the reaction conditions to higher temperatures and used no solvent for the reaction. Further improvement of the reaction was done in the following years, for example the use of ionic liquid
s as solvent for the reaction.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
in which an aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
reacts with cuprous cyanide to an aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
nitrile
Nitrile
A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
..
The reaction was named after Karl Wilhelm Rosenmund
Karl Wilhelm Rosenmund
Karl Wilhelm Rosenmund was a German chemist. He was born in Berlin and died in Kiel.Rosenmund studied chemistry and received his Ph.D. 1906 from University of Berlin for his work with Otto Paul Hermann Diels. He discovered the Rosenmund reduction, which is the reduction of acyl chlorides to...
who together with his Ph.D. student Erich Struck discovered in 1914 that aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
reacts with alcohol water solution of potassium cyanide
Potassium cyanide
Potassium cyanide is an inorganic compound with the formula KCN. This colorless crystalline compound, similar in appearance to sugar, is highly soluble in water. Most KCN is used in gold mining, organic synthesis, and electroplating. Smaller applications include jewelry for chemical gilding and...
and catalytic amounts of cuprous cyanide at 200°C. The reaction yields the carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
, not the nitrile, but Rosenmund speculated that the intermediate should be the nitrile. Independently Alfred Pongratz and Julius von Braun improved the reaction by changing the reaction conditions to higher temperatures and used no solvent for the reaction. Further improvement of the reaction was done in the following years, for example the use of ionic liquid
Ionic liquid
An ionic liquid is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below some arbitrary temperature, such as . While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ILs are largely made...
s as solvent for the reaction.