Carnitine palmitoyltransferase I
Encyclopedia
Carnitine palmitoyltransferase I (CPT1) also known as carnitine acyltransferase I or CAT1 is a mitochondrial
enzyme
. It is part of a family of enzymes called carnitine acyltransferases. Three isoforms
of CPT1 are currently known: CPT1A, CPT1B, and CPT1C. CPT1 is associated with the outer mitochondrial membrane
and mediates the transport of long-chain fatty acids across the membrane by binding them to carnitine
. This enzyme can be inhibited by malonyl CoA. Its role in fatty acid metabolism
makes CPT1 important in many metabolic disorders such as diabetes
. Since its crystal structure
is not known, its exact mechanism of action remains to be elucidated.
that associates with the mitochondrial outer membrane through transmembrane regions in the peptide
chain. Both the N- and C-terminal domains are exposed to the cytosol
ic side of the membrane.
Three isoforms of CPT1 exist in mammalian tissues. The liver isoform (CPT1A or CPTI-L) is found throughout the body on the mitochondria of all cells except for skeletal muscle cells and white and brown adipose cells
. The muscle isoform (CPT1B or CPTI-M) is highly expressed in heart and skeletal muscle cells and white and brown adipose cells. A third isoform, the brain isoform (CPT1C), was isolated in 2002. It is expressed predominantly in the brain and testes. Little is known about CPT1C.
The exact structure of any of the CPT1 isoforms has not yet been determined, although a variety of in silico
models for CPT1 have been created based on closely related carnitine acyltransferases, such as carnitine acetyltransferase (CRAT)
.
An important structural difference between CPT1 and CPT2, CRAT and carnitine octanoyltransferase (COT)
is that CPT1 contains an additional domain at its N-terminal consisting of about 160 amino acids. It has been determined that this additional N-terminal domain is important for the key inhibitory molecule of CPT1, malonyl-CoA.
Two distinct binding site
s have been proposed to exist in CPT1A and CPT1B. The “A site” or “CoA site” appears to bind both malonyl-CoA and palmitoyl-CoA, as well as other molecules containing coenzyme A
, suggesting that the enzyme binds these molecules via interaction with the coenzyme A moiety. It has been suggested that malonyl-CoA may behave as a competitive inhibitor
of CPT1A at this site. A second “O site” has been proposed to bind malonyl-CoA more tightly than the A site. Unlike the A site, the O site binds to malonyl-CoA via the dicarbonyl group of the malonate
moiety of malonyl-CoA. The binding of malonyl-CoA to either the A and O sites inhibits the action of CPT1A by excluding the binding of carnitine to CPT1A. Since a crystal structure of CTP1A has yet to be isolated and imaged, its exact structure remains to be elucidated.
residue
473 as the key catalytic residue. One such mechanism based upon a carnitine acetyltransferase model is shown below in which the His 473 deprotonates carnitine while a nearby serine
residue stabilizes the tetrahedral oxyanion
intermediate.
A different mechanism has been proposed that suggests that a catalytic triad
composed of residues Cys-305, His-473, and Asp-454 carries out the acyl-transferring step of catalysis
. This catalytic mechanism involves the formation of a thioacyl-enzyme covalent
intermediate with Cys-305.
linkage to coenzyme A) on the outer mitochondrial membrane, the activated fatty acids are oxidized within the mitochondrial matrix
. Long chain fatty acids such as palmitoyl-CoA, unlike short- and medium-chain fatty acids, cannot freely diffuse
through the mitochondrial inner membrane, and require a shuttle system to be transported to the mitochondrial matrix.
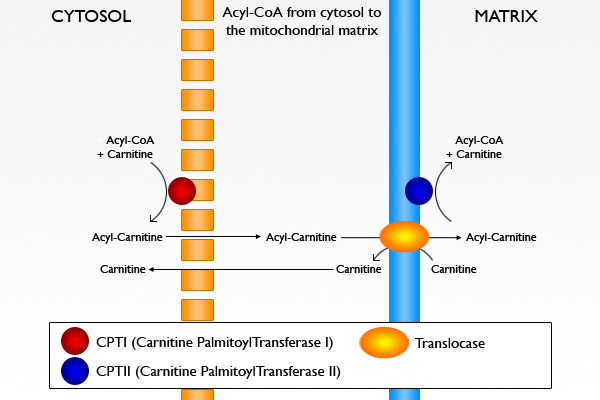 Carnitine palmitoyltransferase I is the first component and rate-limiting step of the carnitine palmitoyltransferase system, catalyzing the transfer of the acyl group from coenzyme A to carnitine to form palmitoylcarnitine
Carnitine palmitoyltransferase I is the first component and rate-limiting step of the carnitine palmitoyltransferase system, catalyzing the transfer of the acyl group from coenzyme A to carnitine to form palmitoylcarnitine
. A translocase
then shuttles the acyl carnitine across the inner mitochondrial membrane where it is converted back into palmitoyl-CoA.
By acting as an acyl group acceptor, carnitine may also play the role of regulating the intracellular CoA:acy-CoA ratio.
Acetyl-CoA carboxylase
(ACC), the enzyme that catalyzes the formation of malonyl-CoA from acetyl-CoA
, is important in the regulation of fatty acid metabolism. Scientists have demonstrated that ACC2 knockout mice
have reduced body fat and weight when compared to wild type
mice. This is a result of decreased activity of ACC which causes a subsequent decrease in malonyl-CoA concentrations. These decreased malonyl-CoA levels in turn prevent inhibition of CPT1, causing an ultimate increase in fatty acid oxidation. Since heart and skeletal muscle cells have a low capacity for fatty acid synthesis, ACC may act purely as a regulatory enzyme in these cells.
. This rare disorder confers risk for hepatic encephalopathy
, hypoketotic hypoglycemia, seizures, and sudden unexpected death in infancy.
CPT1 is associated with type 2 diabetes
and insulin resistance
. Such diseases, along with many other health problems, cause free fatty acid (FFA) levels in humans to become elevated, fat to accumulate in skeletal muscle, and decreases the ability of muscles to oxidize fatty acids. CPT1 has been implicated in playing a critical role in these symptoms. The increased levels of malonyl-CoA caused by hyperglycemia
and hyperinsulinemia
inhibit CPT1, which causes a subsequent decrease in the transport of long chain fatty acids into muscle and heart mitochondria, decreasing fatty acid oxidation in such cells. The shunting of LCFAs away from mitochondria leads to the observed increase in FFA levels and the accumulation of fat in skeletal muscle.
Its importance in fatty acid metabolism makes CPT1 a potentially useful enzyme to focus on in the development of treatments of many other metabolic disorders as well.
Mitochondrion
In cell biology, a mitochondrion is a membrane-enclosed organelle found in most eukaryotic cells. These organelles range from 0.5 to 1.0 micrometers in diameter...
enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
. It is part of a family of enzymes called carnitine acyltransferases. Three isoforms
Protein isoform
A protein isoform is any of several different forms of the same protein. Different forms of a protein may be produced from related genes, or may arise from the same gene by alternative splicing. A large number of isoforms are caused by single-nucleotide polymorphisms or SNPs, small genetic...
of CPT1 are currently known: CPT1A, CPT1B, and CPT1C. CPT1 is associated with the outer mitochondrial membrane
Outer mitochondrial membrane
thumb|300px|Mitochondria structure :1) [[Inner membrane]]2) Outer membrane3) [[Crista]]4) [[Matrix |Matrix]]The outer mitochondrial membrane, which encloses the entire organelle, has a protein-to-phospholipid ratio similar to the eukaryotic plasma membrane...
and mediates the transport of long-chain fatty acids across the membrane by binding them to carnitine
Carnitine
Carnitine is a quaternary ammonium compound biosynthesized from the amino acids lysine and methionine. In living cells, it is required for the transport of fatty acids from the cytosol into the mitochondria during the breakdown of lipids for the generation of metabolic energy. It is widely...
. This enzyme can be inhibited by malonyl CoA. Its role in fatty acid metabolism
Fatty acid metabolism
Fatty acids are an important source of energy and adenosine triphosphate for many cellular organisms. Excess fatty acids, glucose, and other nutrients can be stored efficiently as fat. Triglycerides yield more than twice as much energy for the same mass as do carbohydrates or proteins. All cell...
makes CPT1 important in many metabolic disorders such as diabetes
Diabetes mellitus
Diabetes mellitus, often simply referred to as diabetes, is a group of metabolic diseases in which a person has high blood sugar, either because the body does not produce enough insulin, or because cells do not respond to the insulin that is produced...
. Since its crystal structure
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
is not known, its exact mechanism of action remains to be elucidated.
Structure
CPT1 is an integral membrane proteinIntegral membrane protein
An integral membrane protein is a protein molecule that is permanently attached to the biological membrane. Proteins that cross the membrane are surrounded by "annular" lipids, which are defined as lipids that are in direct contact with a membrane protein...
that associates with the mitochondrial outer membrane through transmembrane regions in the peptide
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
chain. Both the N- and C-terminal domains are exposed to the cytosol
Cytosol
The cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments....
ic side of the membrane.
Three isoforms of CPT1 exist in mammalian tissues. The liver isoform (CPT1A or CPTI-L) is found throughout the body on the mitochondria of all cells except for skeletal muscle cells and white and brown adipose cells
Adipocyte
However, in some reports and textbooks, the number of fat cell increased in childhood and adolescence. The total number is constant in both obese and lean adult...
. The muscle isoform (CPT1B or CPTI-M) is highly expressed in heart and skeletal muscle cells and white and brown adipose cells. A third isoform, the brain isoform (CPT1C), was isolated in 2002. It is expressed predominantly in the brain and testes. Little is known about CPT1C.
The exact structure of any of the CPT1 isoforms has not yet been determined, although a variety of in silico
In silico
In silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...
models for CPT1 have been created based on closely related carnitine acyltransferases, such as carnitine acetyltransferase (CRAT)
Carnitine O-acetyltransferase
In enzymology, a carnitine O-acetyltransferase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are acetyl-CoA and carnitine, whereas its two products are CoA and O-acetylcarnitine....
.
An important structural difference between CPT1 and CPT2, CRAT and carnitine octanoyltransferase (COT)
Carnitine O-octanoyltransferase
In enzymology, a carnitine O-octanoyltransferase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are octanoyl-CoA and L-carnitine, whereas its two products are CoA and L-octanoylcarnitine....
is that CPT1 contains an additional domain at its N-terminal consisting of about 160 amino acids. It has been determined that this additional N-terminal domain is important for the key inhibitory molecule of CPT1, malonyl-CoA.
Two distinct binding site
Binding site
In biochemistry, a binding site is a region on a protein, DNA, or RNA to which specific other molecules and ions—in this context collectively called ligands—form a chemical bond...
s have been proposed to exist in CPT1A and CPT1B. The “A site” or “CoA site” appears to bind both malonyl-CoA and palmitoyl-CoA, as well as other molecules containing coenzyme A
Coenzyme A
Coenzyme A is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All sequenced genomes encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it as a substrate...
, suggesting that the enzyme binds these molecules via interaction with the coenzyme A moiety. It has been suggested that malonyl-CoA may behave as a competitive inhibitor
Competitive inhibition
Competitive inhibition is a form of enzyme inhibition where binding of the inhibitor to the active site on the enzyme prevents binding of the substrate and vice versa.-Mechanism:...
of CPT1A at this site. A second “O site” has been proposed to bind malonyl-CoA more tightly than the A site. Unlike the A site, the O site binds to malonyl-CoA via the dicarbonyl group of the malonate
Malonate
The malonate or propanedioate ion is CH222− . Malonate compounds include salts and esters of malonic acid, such as*diethyl malonate, 2,*dimethyl malonate, 2,...
moiety of malonyl-CoA. The binding of malonyl-CoA to either the A and O sites inhibits the action of CPT1A by excluding the binding of carnitine to CPT1A. Since a crystal structure of CTP1A has yet to be isolated and imaged, its exact structure remains to be elucidated.
Enzyme mechanism
Because crystal structure data is currently unavailable, the exact mechanism of CPT1 is not currently known. A couple different possible mechanisms for CPT1 have been postulated, both of which include the histidineHistidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
residue
Residue (chemistry)
In chemistry, residue is the material remaining after a distillation or an evaporation, or to a portion of a larger molecule, such as a methyl group. It may also refer to the undesired byproducts of a reaction....
473 as the key catalytic residue. One such mechanism based upon a carnitine acetyltransferase model is shown below in which the His 473 deprotonates carnitine while a nearby serine
Serine
Serine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:...
residue stabilizes the tetrahedral oxyanion
Oxyanion
An oxyanion or oxoanion is a chemical compound with the generic formula AxOyz− . Oxoanions are formed by a large majority of the chemical elements. The formulae of simple oxoanions are determined by the octet rule...
intermediate.
A different mechanism has been proposed that suggests that a catalytic triad
Catalytic triad
A catalytic triad refers to the three amino acid residues found inside the active site of certain protease enzymes: serine , aspartate , and histidine . They work together to break peptide bonds on polypeptides. In general terms, catalytic triad can refer to any set of three residues that function...
composed of residues Cys-305, His-473, and Asp-454 carries out the acyl-transferring step of catalysis
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
. This catalytic mechanism involves the formation of a thioacyl-enzyme covalent
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
intermediate with Cys-305.
Biological function
The carnitine palmitoyltransferase system is an essential step in the beta-oxidation of long chain fatty acids. This transfer system is necessary because, while fatty acids are activated (in the form of a thioesterThioester
Thioesters are compounds with the functional group C-S-CO-C. They are the product of esterification between a carboxylic acid and a thiol. Thioesters are widespread in biochemistry, the best-known derivative being acetyl-CoA.-Synthesis:...
linkage to coenzyme A) on the outer mitochondrial membrane, the activated fatty acids are oxidized within the mitochondrial matrix
Mitochondrial matrix
In the mitochondrion, the matrix contains soluble enzymes that catalyze the oxidation of pyruvate and other small organic molecules.The mitochondrial matrix also contains the mitochondria's DNA and ribosomes. The word "matrix" stems from the fact that this space is viscous, compared to the...
. Long chain fatty acids such as palmitoyl-CoA, unlike short- and medium-chain fatty acids, cannot freely diffuse
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
through the mitochondrial inner membrane, and require a shuttle system to be transported to the mitochondrial matrix.

Palmitoylcarnitine
Palmitoylcarnitine is an ester derivative of carnitine involved in the metabolism of fatty acids.Carnitine O-palmitoyltransferase breaks it down into palmitoyl CoA....
. A translocase
Translocase
Translocase is a general term for an enzyme that assists in moving another molecule, usually across a membrane.Translocases are most common secretion system in Gram positive bacteria.Examples include:...
then shuttles the acyl carnitine across the inner mitochondrial membrane where it is converted back into palmitoyl-CoA.
By acting as an acyl group acceptor, carnitine may also play the role of regulating the intracellular CoA:acy-CoA ratio.
Regulation
CPT1 is inhibited by malonyl-CoA, although the exact mechanism of inhibition remains to be known. The CPT1 skeletal muscle and heart isoform, CPT1B, has been shown to be 30-100-fold more sensitive to malonyl-CoA inhibition than CPT1A. This inhibition is a good target for future attempts to regulate CPT1 for the treatment of metabolic disorders.Acetyl-CoA carboxylase
Acetyl-CoA carboxylase
Acetyl-CoA carboxylase is a biotin-dependent enzyme that catalyzes the irreversible carboxylation of acetyl-CoA to produce malonyl-CoA through its two catalytic activities, biotin carboxylase and carboxyltransferase...
(ACC), the enzyme that catalyzes the formation of malonyl-CoA from acetyl-CoA
Acetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
, is important in the regulation of fatty acid metabolism. Scientists have demonstrated that ACC2 knockout mice
Knockout mouse
A knockout mouse is a genetically engineered mouse in which researchers have inactivated, or "knocked out," an existing gene by replacing it or disrupting it with an artificial piece of DNA...
have reduced body fat and weight when compared to wild type
Wild type
Wild type refers to the phenotype of the typical form of a species as it occurs in nature. Originally, the wild type was conceptualized as a product of the standard, "normal" allele at a locus, in contrast to that produced by a non-standard, "mutant" allele...
mice. This is a result of decreased activity of ACC which causes a subsequent decrease in malonyl-CoA concentrations. These decreased malonyl-CoA levels in turn prevent inhibition of CPT1, causing an ultimate increase in fatty acid oxidation. Since heart and skeletal muscle cells have a low capacity for fatty acid synthesis, ACC may act purely as a regulatory enzyme in these cells.
Disease relevance
The "CPT1A" form is associated with carnitine palmitoyltransferase I deficiencyCarnitine palmitoyltransferase I deficiency
Carnitine palmitoyltransferase I deficiency is a rare metabolic disorder that prevents the body from converting certain fats called long-chain fatty acids into energy, particularly during periods without food....
. This rare disorder confers risk for hepatic encephalopathy
Hepatic encephalopathy
Hepatic encephalopathy is the occurrence of confusion, altered level of consciousness and coma as a result of liver failure. In the advanced stages it is called hepatic coma or coma hepaticum...
, hypoketotic hypoglycemia, seizures, and sudden unexpected death in infancy.
CPT1 is associated with type 2 diabetes
Diabetes mellitus type 2
Diabetes mellitus type 2formerly non-insulin-dependent diabetes mellitus or adult-onset diabetesis a metabolic disorder that is characterized by high blood glucose in the context of insulin resistance and relative insulin deficiency. Diabetes is often initially managed by increasing exercise and...
and insulin resistance
Insulin resistance
Insulin resistance is a physiological condition where the natural hormone insulin becomes less effective at lowering blood sugars. The resulting increase in blood glucose may raise levels outside the normal range and cause adverse health effects, depending on dietary conditions. Certain cell types...
. Such diseases, along with many other health problems, cause free fatty acid (FFA) levels in humans to become elevated, fat to accumulate in skeletal muscle, and decreases the ability of muscles to oxidize fatty acids. CPT1 has been implicated in playing a critical role in these symptoms. The increased levels of malonyl-CoA caused by hyperglycemia
Hyperglycemia
Hyperglycemia or Hyperglycæmia, or high blood sugar, is a condition in which an excessive amount of glucose circulates in the blood plasma. This is generally a glucose level higher than 13.5mmol/l , but symptoms may not start to become noticeable until even higher values such as 15-20 mmol/l...
and hyperinsulinemia
Hyperinsulinemia
Hyperinsulinemia, or hyperinsulinaemiais a condition which there is excess levels of insulin circulating in the blood than expected relative to the level of glucose. While it is often mistaken for diabetes or hypoglycaemia, hyperinsulinemia can result from a variety of metabolic diseases and...
inhibit CPT1, which causes a subsequent decrease in the transport of long chain fatty acids into muscle and heart mitochondria, decreasing fatty acid oxidation in such cells. The shunting of LCFAs away from mitochondria leads to the observed increase in FFA levels and the accumulation of fat in skeletal muscle.
Its importance in fatty acid metabolism makes CPT1 a potentially useful enzyme to focus on in the development of treatments of many other metabolic disorders as well.

