
Carcerand
Encyclopedia
A carcerand is a host
molecule that completely entraps its guest so that it will not escape even at high temperatures. This type of molecule was first described by Donald J. Cram
in 1985 and is derived from the Latin
carcer, or prison
. The complexes formed by a carcerand with permanently imprisoned guests are called carceplexes.
In contrast hemicarcerands allow guests to enter and exit the cavity at high temperatures but will form stable complexes at ambient temperatures. The complexes formed by a hemicarcerand and a guests are called hemicarceplexes.
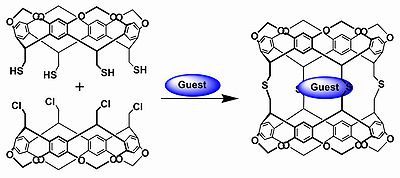 The first generation carcerands are based on calixarene
The first generation carcerands are based on calixarene
hemicarcerands with 4 alkyl substituents on the upper rim and 4 reactive substituent
s on the lower rim. The coupling of both hemicarcerands takes place through a spacer group. In the original 1985 publication two different hemicarcerands react, one with chloromethyl reactive groups and one with thiomethyl reactive groups in a nucleophilic displacement and the resulting the spacer group is a dimethylsulfide (CH2SCH2). In this experiment the guests were the molecules already present in the reaction medium such as argon
and dimethylformamide
.
In another configuration the 4 lower rim functional groups are aldehyde
s which condense
with O-Phenylenediamine
to the corresponding di-imines. The 4 spacer groups connecting the two spheres are now much longer and consequently the internal cavity is much larger. Compounds trapped in the cavity are said to be held there by constrictive binding. They can be introduced by simply heating in neat solvent like hexachlorobutadiene (a fungicide
). The half-life
of the reverse process is 3.2 hours at 25 °C in CDCl3 by NMR analysis. Ferrocene
can be introduced by heating with the hemicarcerand in a large bulky solvent such as tripiperidylphosphine oxide. The half-life for ferrocene liberation is 19.6 hours at 112 °C.
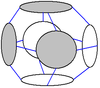 The internal cavity of a carcerand can be as large as 1700 Å
The internal cavity of a carcerand can be as large as 1700 Å
3 (1.7 nm3) when six hemicarcerands form a single octahedral compound. This is accomplished by dynamic covalent chemistry
in a one-pot
condensation of 6 equivalents of a tetraformyl calixarene and 12 equivalents of ethylene diamine
with trifluoroacetic acid
catalyst in chloroform
at room temperature
followed by reduction of the imine bonds with sodium borohydride
.
Host-guest chemistry
In supramolecular chemistry, host-guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host-guest chemistry encompasses the idea of molecular recognition...
molecule that completely entraps its guest so that it will not escape even at high temperatures. This type of molecule was first described by Donald J. Cram
Donald J. Cram
Donald James Cram was an American chemist who shared the 1987 Nobel Prize in Chemistry with Jean-Marie Lehn and Charles J...
in 1985 and is derived from the Latin
Latin
Latin is an Italic language originally spoken in Latium and Ancient Rome. It, along with most European languages, is a descendant of the ancient Proto-Indo-European language. Although it is considered a dead language, a number of scholars and members of the Christian clergy speak it fluently, and...
carcer, or prison
Prison
A prison is a place in which people are physically confined and, usually, deprived of a range of personal freedoms. Imprisonment or incarceration is a legal penalty that may be imposed by the state for the commission of a crime...
. The complexes formed by a carcerand with permanently imprisoned guests are called carceplexes.
In contrast hemicarcerands allow guests to enter and exit the cavity at high temperatures but will form stable complexes at ambient temperatures. The complexes formed by a hemicarcerand and a guests are called hemicarceplexes.
Reactivity of bound guests
Cram described the interior of the container compound as the inner phase in which radically different reactivity was observed. He used a hemicarcerand to isolate highly unstable, antiaromatic cylobutadiene at room temperature. The hemicarcerand stabilizes guests within its cavity by preventing their reaction with other molecules.Synthesis

Calixarene
A calixarene is a macrocycle or cyclic oligomer based on a hydroxyalkylation product of a phenol and an aldehyde. The word calixarene is derived from calix or chalice because this type of molecule resembles a vase and from the word arene that refers to the aromatic building block...
hemicarcerands with 4 alkyl substituents on the upper rim and 4 reactive substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s on the lower rim. The coupling of both hemicarcerands takes place through a spacer group. In the original 1985 publication two different hemicarcerands react, one with chloromethyl reactive groups and one with thiomethyl reactive groups in a nucleophilic displacement and the resulting the spacer group is a dimethylsulfide (CH2SCH2). In this experiment the guests were the molecules already present in the reaction medium such as argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
and dimethylformamide
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
.
In another configuration the 4 lower rim functional groups are aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s which condense
Alkylimino-de-oxo-bisubstitution
Alkylimino-de-oxo-bisubstitution in organic chemistry is the organic reaction of carbonyl compounds with amines to imines . The reaction name is based on the IUPAC Nomenclature for Transformations...
with O-Phenylenediamine
O-Phenylenediamine
o-Phenylenediamine is a organic compound with the formula C6H42. This aromatic diamine is an important precursor to many heterocyclic compounds...
to the corresponding di-imines. The 4 spacer groups connecting the two spheres are now much longer and consequently the internal cavity is much larger. Compounds trapped in the cavity are said to be held there by constrictive binding. They can be introduced by simply heating in neat solvent like hexachlorobutadiene (a fungicide
Fungicide
Fungicides are chemical compounds or biological organisms used to kill or inhibit fungi or fungal spores. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality and profit. Fungicides are used both in agriculture and to fight fungal infections in animals...
). The half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of the reverse process is 3.2 hours at 25 °C in CDCl3 by NMR analysis. Ferrocene
Ferrocene
Ferrocene is an organometallic compound with the formula Fe2. It is the prototypical metallocene, a type of organometallic chemical compound consisting of two cyclopentadienyl rings bound on opposite sides of a central metal atom. Such organometallic compounds are also known as sandwich compounds...
can be introduced by heating with the hemicarcerand in a large bulky solvent such as tripiperidylphosphine oxide. The half-life for ferrocene liberation is 19.6 hours at 112 °C.
Large Carcerands

Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
3 (1.7 nm3) when six hemicarcerands form a single octahedral compound. This is accomplished by dynamic covalent chemistry
Dynamic covalent chemistry
In supramolecular chemistry, dynamic covalent chemistry is a strategy that aims at synthesizing large complex molecules. In it a reversible reaction is under thermodynamic reaction control and a specific reaction product out of many is captured...
in a one-pot
One-pot synthesis
In chemistry a one-pot synthesis is a strategy to improve the efficiency of a chemical reaction whereby a reactant is subjected to successive chemical reactions in just one reactor...
condensation of 6 equivalents of a tetraformyl calixarene and 12 equivalents of ethylene diamine
Ethylene diamine
Ethylenediamine is the organic compound with the formula C2H42. This colorless liquid with an ammonia-like odor is a strongly basic amine. The liquid fumes upon contact with humid air...
with trifluoroacetic acid
Trifluoroacetic acid
Trifluoroacetic acid is the simplest stable perfluorinated carboxylic acid chemical compound, with the formula CF3CO2H. It is a strong carboxylic acid due to the influence of the electronegative trifluoromethyl group. TFA is almost 100,000-fold more acidic than acetic acid...
catalyst in chloroform
Chloroform
Chloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous...
at room temperature
Room temperature
-Comfort levels:The American Society of Heating, Refrigerating and Air-Conditioning Engineers has listings for suggested temperatures and air flow rates in different types of buildings and different environmental circumstances. For example, a single office in a building has an occupancy ratio per...
followed by reduction of the imine bonds with sodium borohydride
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
.

