Carbon dioxide in the Earth's atmosphere
Encyclopedia
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
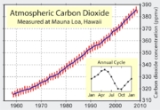
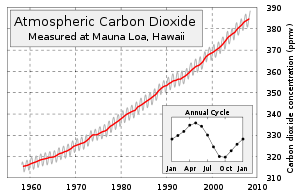
in Earth's atmosphere is approximately 392 ppm (parts per million) by volume and rose by 2.0 ppm/yr during 2000–2009. 40 years earlier, the rise was only 0.9 ppm/yr, showing not only increasing concentrations, but also a rapid acceleration of concentrations. The increase of concentration from pre-industrial concentrations has again doubled in just the last 31 years.http://www.esrl.noaa.gov/gmd/ccgg/trends/weekly.html Carbon dioxide is essential to photosynthesis
Photosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
in plant
Plant
Plants are living organisms belonging to the kingdom Plantae. Precise definitions of the kingdom vary, but as the term is used here, plants include familiar organisms such as trees, flowers, herbs, bushes, grasses, vines, ferns, mosses, and green algae. The group is also called green plants or...
s and other photoautotrophs, and is also a prominent greenhouse gas
Greenhouse gas
A greenhouse gas is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. This process is the fundamental cause of the greenhouse effect. The primary greenhouse gases in the Earth's atmosphere are water vapor, carbon dioxide, methane, nitrous oxide, and ozone...
. Despite its relatively small overall concentration in the atmosphere, is an important component of Earth's atmosphere because it absorbs and emits infrared
Infrared
Infrared light is electromagnetic radiation with a wavelength longer than that of visible light, measured from the nominal edge of visible red light at 0.74 micrometres , and extending conventionally to 300 µm...
radiation at wavelength
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
s of 4.26 µm (asymmetric stretching vibrational mode
Infrared spectroscopy
Infrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
) and 14.99 µm (bending vibrational mode), thereby playing a role in the greenhouse effect
Greenhouse effect
The greenhouse effect is a process by which thermal radiation from a planetary surface is absorbed by atmospheric greenhouse gases, and is re-radiated in all directions. Since part of this re-radiation is back towards the surface, energy is transferred to the surface and the lower atmosphere...
in addition to other factors such as water vapour. The present level is higher than at any time during the last 800 thousand years, and likely higher than in the past 20 million years.
Current concentration
In 2009, the global average concentration in Earth's atmosphereEarth's atmosphere
The atmosphere of Earth is a layer of gases surrounding the planet Earth that is retained by Earth's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention , and reducing temperature extremes between day and night...
was about 0.0387% by volume, or 387 parts per million by volume (ppmv)
Parts-per notation
In science and engineering, the parts-per notation is a set of pseudo units to describe small values of miscellaneous dimensionless quantities, e.g. mole fraction or mass fraction. Since these fractions are quantity-per-quantity measures, they are pure numbers with no associated units of measurement...
. There is an annual fluctuation of about 3–9 ppmv which roughly follows the Northern Hemisphere's growing season. The Northern Hemisphere
Northern Hemisphere
The Northern Hemisphere is the half of a planet that is north of its equator—the word hemisphere literally means “half sphere”. It is also that half of the celestial sphere north of the celestial equator...
dominates the annual cycle of concentration because it has much greater land area and plant biomass than the Southern Hemisphere. Concentrations peak in May as the Northern Hemisphere spring greenup begins and reach a minimum in October when the quantity of biomass
Biomass
Biomass, as a renewable energy source, is biological material from living, or recently living organisms. As an energy source, biomass can either be used directly, or converted into other energy products such as biofuel....
undergoing photosynthesis is greatest.
Sources of carbon dioxide
Natural sources of atmospheric carbon dioxide include volcanic outgassing
Outgassing
Outgassing is the release of a gas that was dissolved, trapped, frozen or absorbed in some material. As an example, research has shown how the concentration of carbon dioxide in the Earth's atmosphere has sometimes been linked to ocean outgassing...
, the combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
of organic matter
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
, and the respiration
Respiration (physiology)
'In physiology, respiration is defined as the transport of oxygen from the outside air to the cells within tissues, and the transport of carbon dioxide in the opposite direction...
processes of living aerobic organism
Aerobic organism
An aerobic organism or aerobe is an organism that can survive and grow in an oxygenated environment.Faculitative anaerobes grow and survive in an oxygenated environment and so do aerotolerant anaerobes.-Glucose:...
s; man-made sources of carbon dioxide include the burning of fossil fuels for heating, power generation
Electricity generation
Electricity generation is the process of generating electric energy from other forms of energy.The fundamental principles of electricity generation were discovered during the 1820s and early 1830s by the British scientist Michael Faraday...
and transport
Transport
Transport or transportation is the movement of people, cattle, animals and goods from one location to another. Modes of transport include air, rail, road, water, cable, pipeline, and space. The field can be divided into infrastructure, vehicles, and operations...
, as well as some industrial processes such as cement making. It is also produced by various microorganism
Microorganism
A microorganism or microbe is a microscopic organism that comprises either a single cell , cell clusters, or no cell at all...
s from fermentation
Fermentation (biochemistry)
Fermentation is the process of extracting energy from the oxidation of organic compounds, such as carbohydrates, using an endogenous electron acceptor, which is usually an organic compound. In contrast, respiration is where electrons are donated to an exogenous electron acceptor, such as oxygen,...
and cellular respiration
Cellular respiration
Cellular respiration is the set of the metabolic reactions and processes that take place in the cells of organisms to convert biochemical energy from nutrients into adenosine triphosphate , and then release waste products. The reactions involved in respiration are catabolic reactions that involve...
. Plant
Plant
Plants are living organisms belonging to the kingdom Plantae. Precise definitions of the kingdom vary, but as the term is used here, plants include familiar organisms such as trees, flowers, herbs, bushes, grasses, vines, ferns, mosses, and green algae. The group is also called green plants or...
s convert carbon dioxide to carbohydrate
Carbohydrate
A carbohydrate is an organic compound with the empirical formula ; that is, consists only of carbon, hydrogen, and oxygen, with a hydrogen:oxygen atom ratio of 2:1 . However, there are exceptions to this. One common example would be deoxyribose, a component of DNA, which has the empirical...
s during a process called photosynthesis
Photosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
. They gain the energy needed for this reaction through the absorption of sunlight by pigments such as Chlorophyll
Chlorophyll
Chlorophyll is a green pigment found in almost all plants, algae, and cyanobacteria. Its name is derived from the Greek words χλωρος, chloros and φύλλον, phyllon . Chlorophyll is an extremely important biomolecule, critical in photosynthesis, which allows plants to obtain energy from light...
. The resulting gas, oxygen, is released into the atmosphere by plants, which is subsequently used for respiration by heterotrophic organisms and other plants, forming a cycle
Carbon cycle
The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth...
.
Most sources of emissions are natural. For example, the natural decay of organic material in forests and grasslands, such as dead trees, results in the release of about 220 gigatonnes of carbon dioxide every year. In 1997, human-caused Indonesian peat fires
1997 Southeast Asian haze
The 1997 Southeast Asian haze was a large-scale air quality disaster which occurred during the second half of 1997, its after-effects causing widespread atmospheric visibility and health problems within Southeast Asia...
were estimated to have released between 13% and 40% of the average carbon emissions caused by the burning of fossil fuels around the world in a single year. Although the initial carbon dioxide in the atmosphere of the young Earth was produced by volcanic activity
Volcano
2. Bedrock3. Conduit 4. Base5. Sill6. Dike7. Layers of ash emitted by the volcano8. Flank| 9. Layers of lava emitted by the volcano10. Throat11. Parasitic cone12. Lava flow13. Vent14. Crater15...
, modern volcanic activity releases only 130 to 230 megatonnes
Tonne
The tonne, known as the metric ton in the US , often put pleonastically as "metric tonne" to avoid confusion with ton, is a metric system unit of mass equal to 1000 kilograms. The tonne is not an International System of Units unit, but is accepted for use with the SI...
of carbon dioxide each year, which is less than 1% of the amount released by human activities.
These natural sources are nearly balanced by natural sinks, physical and biological processes which remove carbon dioxide from the atmosphere. For example, some is directly removed from the atmosphere by land plants for photosynthesis
Photosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
and it is soluble in water forming carbonic acid
Carbonic acid
Carbonic acid is the inorganic compound with the formula H2CO3 . It is also a name sometimes given to solutions of carbon dioxide in water, because such solutions contain small amounts of H2CO3. Carbonic acid forms two kinds of salts, the carbonates and the bicarbonates...
.
There is a large natural flux of into and out of the biosphere and oceans. In the pre-industrial era these fluxes were largely in balance. Currently about 57% of human-emitted is removed by the biosphere and oceans. The ratio of the increase in atmospheric to emitted is known as the airborne fraction (Keeling et al., 1995); this varies for short-term averages but is typically about 45% over longer (5 year) periods. Estimated carbon in global terrestrial vegetation increased from approximately 740 billion tons in 1910 to 780 billion tons in 1990.
Burning fossil fuels such as coal
Coal
Coal is a combustible black or brownish-black sedimentary rock usually occurring in rock strata in layers or veins called coal beds or coal seams. The harder forms, such as anthracite coal, can be regarded as metamorphic rock because of later exposure to elevated temperature and pressure...
and petroleum
Petroleum
Petroleum or crude oil is a naturally occurring, flammable liquid consisting of a complex mixture of hydrocarbons of various molecular weights and other liquid organic compounds, that are found in geologic formations beneath the Earth's surface. Petroleum is recovered mostly through oil drilling...
is the leading cause of increased anthropogenic ; deforestation
Deforestation
Deforestation is the removal of a forest or stand of trees where the land is thereafter converted to a nonforest use. Examples of deforestation include conversion of forestland to farms, ranches, or urban use....
is the second major cause. In 2008, 8.67 gigatonnes of carbon (31.8 gigatonnes of ) were released from fossil fuels worldwide, compared to 6.14 gigatonnes in 1990. In addition, land use change contributed 1.20 gigatonnes in 2008, compared to 1.64 gigatonnes in 1990.
In the period 1751 to 1900 about 12 gigatonnes of carbon were released as carbon dioxide to the atmosphere from burning of fossil fuels, whereas from 1901 to 2008 the figure was about 334 gigatonnes.
This addition, about 3% of annual natural emissions , is sufficient to exceed the balancing effect of sinks. As a result, carbon dioxide has gradually accumulated in the atmosphere, and , its concentration is 39% above pre-industrial levels.
Various techniques have been proposed for removing excess carbon dioxide from the atmosphere in carbon dioxide sink
Carbon dioxide sink
A carbon sink is a natural or artificial reservoir that accumulates and stores some carbon-containing chemical compound for an indefinite period. The process by which carbon sinks remove carbon dioxide from the atmosphere is known as carbon sequestration...
s.
Past variation
The most direct method for measuring atmospheric carbon dioxide concentrations for periods before direct sampling is to measure bubbles of air (fluid or gas inclusionsFluid inclusions
thumb|250px|Trapped in a time capsule the same size as the diameter of a human hair, the ore-forming liquid in this inclusion was so hot and contained so much dissolved solids that when it cooled, crystals of halite, sylvite, gypsum, and hematite formed. As the samples cooled, the fluid shrank more...
) trapped in the Antarctic or Greenland
Greenland
Greenland is an autonomous country within the Kingdom of Denmark, located between the Arctic and Atlantic Oceans, east of the Canadian Arctic Archipelago. Though physiographically a part of the continent of North America, Greenland has been politically and culturally associated with Europe for...
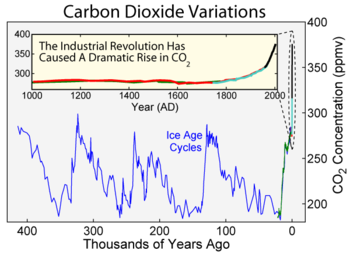
ice caps. The most widely accepted of such studies come from a variety of Antarctic cores and indicate that atmospheric levels were about 260–280 ppmv immediately before industrial emissions began and did not vary much from this level during the preceding 10,000 years (10 ka). In 1832 Antarctic ice core levels were 284 ppmv.
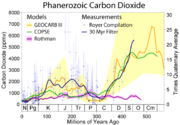
Calcium carbonate
Calcium carbonate is a chemical compound with the formula CaCO3. It is a common substance found in rocks in all parts of the world, and is the main component of shells of marine organisms, snails, coal balls, pearls, and eggshells. Calcium carbonate is the active ingredient in agricultural lime,...
dust found in the ice. When dust levels in Greenland cores are low, as they nearly always are in Antarctic cores, the researchers report good agreement between Antarctic and Greenland measurements.
The longest ice core
Ice core
An ice core is a core sample that is typically removed from an ice sheet, most commonly from the polar ice caps of Antarctica, Greenland or from high mountain glaciers elsewhere. As the ice forms from the incremental build up of annual layers of snow, lower layers are older than upper, and an ice...
record comes from East Antarctica, where ice has been sampled to an age of 800 ka. During this time, the atmospheric carbon dioxide concentration has varied by volume between 180–210 ppm during ice age
Ice age
An ice age or, more precisely, glacial age, is a generic geological period of long-term reduction in the temperature of the Earth's surface and atmosphere, resulting in the presence or expansion of continental ice sheets, polar ice sheets and alpine glaciers...
s, increasing to 280–300 ppm during warmer interglacial
Interglacial
An Interglacial period is a geological interval of warmer global average temperature lasting thousands of years that separates consecutive glacial periods within an ice age...
s. The beginning of human agriculture during the current Holocene
Holocene
The Holocene is a geological epoch which began at the end of the Pleistocene and continues to the present. The Holocene is part of the Quaternary period. Its name comes from the Greek words and , meaning "entirely recent"...
epoch may have been strongly connected to the atmospheric increase after the last ice age ended, a fertilization effect raising plant biomass growth and reducing stoma
Stoma
In botany, a stoma is a pore, found in the leaf and stem epidermis that is used forgas exchange. The pore is bordered by a pair of specialized parenchyma cells known as guard cells that are responsible for regulating the size of the opening...
tal conductance requirements for intake, consequently reducing transpiration water losses and increasing water usage efficiency.
On long timescales, atmospheric content is determined by the balance among geochemical processes including organic carbon burial in sediments, silicate rock weathering
Weathering
Weathering is the breaking down of rocks, soils and minerals as well as artificial materials through contact with the Earth's atmosphere, biota and waters...
, and volcanism. The net effect of slight imbalances in the carbon cycle
Carbon cycle
The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth...
over tens to hundreds of millions of years has been to reduce atmospheric . The rates of these processes are extremely slow; hence they are of limited relevance to the atmospheric response to emissions over the next hundred years.
Various proxy measurements
Proxy (climate)
In the study of past climates is known as paleoclimatology, climate proxies are preserved physical characteristics of the past that stand in for direct measurements , to enable scientists to reconstruct the climatic conditions that prevailed during much of the Earth's history...
have been used to attempt to determine atmospheric carbon dioxide levels millions of years in the past. These include boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
and carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
ratios in certain types of marine sediments, and the number of stomata observed on fossil plant leaves. While these measurements give much less precise estimates of carbon dioxide concentration than ice cores, there is evidence for very high volume concentrations between 200 and 150 Ma of over 3,000 ppm and between 600 and 400 Ma of over 6,000 ppm. In more recent times, atmospheric concentration continued to fall after about 60 Ma. About 34 Ma, the time of the Eocene-Oligocene extinction event
Eocene-Oligocene extinction event
The transition between the end of the Eocene and the beginning of the Oligocene, called the Grande Coupure in Europe, occurring 33.9 ± 0.1 Ma, is marked by large-scale extinction and floral and faunal turnover .Most of the affected organisms were marine or aquatic in nature...
and when the Antarctic ice sheet
Antarctic ice sheet
The Antarctic ice sheet is one of the two polar ice caps of the Earth. It covers about 98% of the Antarctic continent and is the largest single mass of ice on Earth. It covers an area of almost 14 million square km and contains 30 million cubic km of ice...
started to take its current form, is found to have been about 760 ppm, and there is geochemical evidence that volume concentrations were less than 300 ppm by about 20 Ma. Low concentrations may have been the stimulus that favored the evolution of C4
C4 carbon fixation
C4 carbon fixation is one of three biochemical mechanisms, along with and CAM photosynthesis, used in carbon fixation. It is named for the 4-carbon molecule present in the first product of carbon fixation in these plants, in contrast to the 3-carbon molecule products in plants. fixation is an...
plants, which increased greatly in abundance between 7 and 5 Ma.
Relationship with oceanic concentration
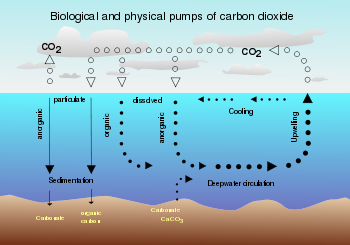
Ocean
An ocean is a major body of saline water, and a principal component of the hydrosphere. Approximately 71% of the Earth's surface is covered by ocean, a continuous body of water that is customarily divided into several principal oceans and smaller seas.More than half of this area is over 3,000...
s contain a huge amount of carbon dioxide in the form of bicarbonate and carbonate ions — much more than the amount in the atmosphere. The bicarbonate is produced in reactions between rock, water, and carbon dioxide. One example is the dissolution of calcium carbonate:
- + + + 2
Reactions like this tend to buffer changes in atmospheric . Since the right-hand side of the reaction produces an acidic compound, adding on the left-hand side decreases the pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
of sea water, a process which has been termed ocean acidification
Ocean acidification
Ocean acidification is the name given to the ongoing decrease in the pH and increase in acidity of the Earth's oceans, caused by the uptake of anthropogenic carbon dioxide from the atmosphere....
(even though pH remains alkaline). Reactions between carbon dioxide and non-carbonate rocks also add bicarbonate to the seas. This can later undergo the reverse of the above reaction to form carbonate rocks, releasing half of the bicarbonate as . Over hundreds of millions of years this has produced huge quantities of carbonate rocks.
Ultimately, most of the emitted by human activities will dissolve in the ocean; however, the rate at which the ocean will take it up in the future is less certain.
Even if equilibrium is reached, including dissolution of carbonate minerals, the increased concentration of bicarbonate and decreased or unchanged concentration of carbonate ion will give rise to a higher concentration of un-ionized carbonic acid and dissolved carbon dioxide gas. This, along with higher temperatures, would mean a higher equilibrium concentration of carbon dioxide in the air.
Irreversibility and uniqueness of carbon dioxide
Carbon dioxide has unique long-term effects on climate change that are largely "irreversible" for one thousand years after emissions stop (zero further emissions) even though carbon dioxide tends toward equilibrium with the ocean on a scale of 100 years. The greenhouse gases methaneMethane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
and nitrous oxide
Nitrous oxide
Nitrous oxide, commonly known as laughing gas or sweet air, is a chemical compound with the formula . It is an oxide of nitrogen. At room temperature, it is a colorless non-flammable gas, with a slightly sweet odor and taste. It is used in surgery and dentistry for its anesthetic and analgesic...
do not persist over time in the same way as carbon dioxide. Even if carbon emissions were to completely cease, atmospheric temperatures are not expected to decrease significantly.
Carbon dioxide management
Carbon dioxide concentrations are growing rapidly and accelerating. The observed concentration rise is through multiple lines of evidence directly attributable to the use of gas, oil and coal. Of any emitted carbon dioxide, about 40% remains semipermanent in the atmosphere. According to a 2007 report by the Intergovernmental Panel on Climate Change, "About 50% of a increase will be removed from the atmosphere within 30 years, and a further 30% will be removed within a few centuries. The remaining 20% may stay in the atmosphere for many thousands of years."Three longer term processes are recognized to redistribute and eventually dissipate currently emitted carbon dioxide. The first will be ocean invasion (300 years), which can only reduce concentration by a factor of ~4, because of the establishment of a new equilibrium. The second will be a new equilibrium with calcium carbonate, which can reduce the concentration by a factor of ~3 over a 5,000-year timescale. The third stage is eventual reaction with igneous rock with a time-constant of 400,000 years. These processes are so slow, that practically zero-emissions are at some point unavoidable in order not to exceed any practical carbon dioxide concentration limit.
To avoid a global warming
Global warming
Global warming refers to the rising average temperature of Earth's atmosphere and oceans and its projected continuation. In the last 100 years, Earth's average surface temperature increased by about with about two thirds of the increase occurring over just the last three decades...
of 2.1°C, it is estimated that a concentration of less than 450 ppm needs to be maintained if other gasses were to return to pre-industrial levels. Currently, a global warming of 0.7°C is measured, with another 0.6°C increase expected even without any further increased concentrations because the oceans are still being warmed along with the atmosphere. At the current accelerated growth rate, exponentially extrapolating the Keeling curve
Keeling curve
The Keeling Curve is a graph which plots the ongoing change in concentration of carbon dioxide in Earth's atmosphere since 1958. It is based on continuous measurements taken at the Mauna Loa Observatory in Hawaii under the supervision of Charles David Keeling. Keeling's measurements showed the...
, this concentration will be reached in 22 years. Even with constant concentration growth, with the current 2.2 ppm/yr, this concentration will be reached in (450-390 ppm)/(2.2 ppm/yr)=27 years. These timescales are so short with respect to the timescale of the evolution that there is little doubt these concentrations will be reached soon barring any drastic behavior changes. The lifetime of power plants for instance can be 40 to 60 years. To avoid dangerous climate change
Avoiding Dangerous Climate Change
The related terms "avoiding dangerous climate change" and "preventing dangerous anthropogenic interference with the climate system" date to 1995 and earlier, in the Second Assesment Report of the International Panel on Climate Change and previous science it cites.In 2002, the United Nations...
, a reduction of the concentration increase of 3.5% per year needs to be achieved for the foreseeable future. Reducing the concentration increase can be done by restricting emissions or with carbon sequestration. The concentration increase is dominated by human emissions.
The current increase to 386 ppm from 280 ppm causes a radiative forcing of 1.66 W/m2, and 1.34 W/m2 from increases in other gases, totaling 3.00 W/m2. The current concentration of greenhouse gases already has a heating power equaling that of a concentration of (386−280)×3.00/1.66 + 280 = 472 ppm C02-eq (carbon dioxide equivalent). Therefore, the current concentrations are high enough for a temperature rise of more than 2° C.
To be able to reduce carbon dioxide concentration by Carbon sequestration back to pre-industrial levels, (390−280 ppm)/390ppm/(50%/100) = 70% of all the CO2 in the air needs to be removed, where 50% is the percentage of carbon dioxide residing in the atmosphere (and not in the oceans), removing about (390−280 ppm)/(50%/100) = 0.03% of the air — an immense task.
See also
- Avoiding Dangerous Climate ChangeAvoiding Dangerous Climate ChangeThe related terms "avoiding dangerous climate change" and "preventing dangerous anthropogenic interference with the climate system" date to 1995 and earlier, in the Second Assesment Report of the International Panel on Climate Change and previous science it cites.In 2002, the United Nations...
— A Scientific Symposium on Stabilisation of Greenhouse Gases - Carbon cycleCarbon cycleThe carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth...
- Carbon dioxide equivalentCarbon dioxide equivalentCarbon dioxide equivalent and Equivalent carbon dioxide are two related but distinct measures for describing how much global warming a given type and amount of greenhouse gas may cause, using the functionally equivalent amount or concentration of carbon dioxide as the reference.- Global warming...
- Climate changeClimate changeClimate change is a significant and lasting change in the statistical distribution of weather patterns over periods ranging from decades to millions of years. It may be a change in average weather conditions or the distribution of events around that average...
- Eddy covarianceEddy covarianceThe eddy covariance technique is a key atmospheric flux measurement technique to measure and calculate vertical turbulent fluxes within atmospheric boundary layers...
flux (aka eddy correlation, eddy flux) - Global warmingGlobal warmingGlobal warming refers to the rising average temperature of Earth's atmosphere and oceans and its projected continuation. In the last 100 years, Earth's average surface temperature increased by about with about two thirds of the increase occurring over just the last three decades...
- Greenhouse effectGreenhouse effectThe greenhouse effect is a process by which thermal radiation from a planetary surface is absorbed by atmospheric greenhouse gases, and is re-radiated in all directions. Since part of this re-radiation is back towards the surface, energy is transferred to the surface and the lower atmosphere...
- List of countries by carbon dioxide emissions per capita
- List of countries by carbon dioxide emissions
- List of countries by ratio of GDP to carbon dioxide emissions
- Ocean acidificationOcean acidificationOcean acidification is the name given to the ongoing decrease in the pH and increase in acidity of the Earth's oceans, caused by the uptake of anthropogenic carbon dioxide from the atmosphere....

