
Aquaporin
Encyclopedia
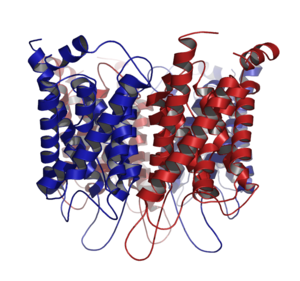
Aquaporins are integral membrane protein
Integral membrane protein
An integral membrane protein is a protein molecule that is permanently attached to the biological membrane. Proteins that cross the membrane are surrounded by "annular" lipids, which are defined as lipids that are in direct contact with a membrane protein...
s from a larger family
Protein family
A protein family is a group of evolutionarily-related proteins, and is often nearly synonymous with gene family. The term protein family should not be confused with family as it is used in taxonomy....
of major intrinsic proteins
Major intrinsic proteins
Major intrinsic proteins are a large family of transmembrane protein channels that are grouped together on the basis of sequence similarities. Proteins from this family exhibit essentially two distinct types of channel properties: specific water...
(MIP) that form pore
Pore
- Animal biology and microbiology :* Sweat pore, an anatomical structure of the skin of humans used for secretion of sweat* Canal pore, an anatomical structure that is part of the lateral line sense system of some aquatic organisms...
s in the membrane
Cell membrane
The cell membrane or plasma membrane is a biological membrane that separates the interior of all cells from the outside environment. The cell membrane is selectively permeable to ions and organic molecules and controls the movement of substances in and out of cells. It basically protects the cell...
of biological cell
Cell (biology)
The cell is the basic structural and functional unit of all known living organisms. It is the smallest unit of life that is classified as a living thing, and is often called the building block of life. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos....
s.
Genetic defects involving aquaporin gene
Gene
A gene is a molecular unit of heredity of a living organism. It is a name given to some stretches of DNA and RNA that code for a type of protein or for an RNA chain that has a function in the organism. Living beings depend on genes, as they specify all proteins and functional RNA chains...
s have been associated with several human diseases. The 2003 Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
was awarded jointly to Peter Agre
Peter Agre
Peter Agre is an American medical doctor, professor, and molecular biologist who was awarded the 2003 Nobel Prize in Chemistry for his discovery of aquaporins. Aquaporins are water-channel proteins that move water molecules through the cell membrane...
for the discovery of aquaporins, and Roderick MacKinnon
Roderick MacKinnon
Roderick MacKinnon is a professor of Molecular Neurobiology and Biophysics at Rockefeller University who won the Nobel Prize in Chemistry together with Peter Agre in 2003 for his work on the structure and operation of ion channels....
for his work on the structure and mechanism of potassium channel
Potassium channel
In the field of cell biology, potassium channels are the most widely distributed type of ion channel and are found in virtually all living organisms. They form potassium-selective pores that span cell membranes...
s.
Function
Aquaporins are "the plumbing system for cells," said Agre. Every cell is primarily water. "But the water doesn’t just sit in the cell, it moves through it in a very organized way. The process occurs rapidly in tissues that have these aquaporins or water channels."For many years, scientists assumed that water leaked through the cell membrane, and some water does. "But the very rapid movement of water through some cells was not explained by this theory," said Agre.
Aquaporins selectively conduct water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s in and out of the cell, while preventing the passage of ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s and other solute
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
s. Also known as water channels, aquaporins are integral membrane pore proteins. Some of them, known as aquaglyceroporins, transport also other small uncharged solutes, such as glycerol
Glycerol
Glycerol is a simple polyol compound. It is a colorless, odorless, viscous liquid that is widely used in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature. The glycerol backbone is central to all lipids...
, CO2, ammonia and urea across the membrane, depending on the size of the pore. However, the water pores are completely impermeable to charged species, such as protons, a property critical for the conservation of the membrane's electrochemical potential.
Water molecules traverse through the pore of the channel in single file. The presence of water channels increases membrane permeability to water.
Many human cell types express them, as do certain bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
and many other organism
Organism
In biology, an organism is any contiguous living system . In at least some form, all organisms are capable of response to stimuli, reproduction, growth and development, and maintenance of homoeostasis as a stable whole.An organism may either be unicellular or, as in the case of humans, comprise...
s, such as plants for which it is essential for the water transport system.
Discovery
Agre said he discovered aquaporins "by serendipity." His lab had an N.I.H. grant to study the Rh blood group antigen. They isolated the Rh molecule but a second molecule, 28 kilodaltons in size (and therefore called 28K) kept appearing. At first they thought it was a piece of the Rh molecule, or a contaminant, but it turned out to be an undiscovered molecule with unknown function. It was abundant in red blood cells and kidney tubes, and related to proteins of diverse origins, like the brains of fruit flies, bacteria, the lenses of eyes, and plant tissues.Agre asked John Parker, his hematology professor at the University of North Carolina. Parker said, “Boy, this thing is found in red cells, kidney tubes, plant tissues; have you considered it might be the long-sought water channel?” So Agre said that he followed up Parker's suggestion.
In most cells, water moves in and out by osmosis through the lipid component of cell membranes. Due to the relatively high water permeability of some epithelial cells it was long suspected that some additional mechanism for water transport across membranes must exist. But it was not until 1992 that the first aquaporin, ‘aquaporin-1’ (originally known as CHIP 28), was reported by Peter Agre
Peter Agre
Peter Agre is an American medical doctor, professor, and molecular biologist who was awarded the 2003 Nobel Prize in Chemistry for his discovery of aquaporins. Aquaporins are water-channel proteins that move water molecules through the cell membrane...
, of Johns Hopkins University
Johns Hopkins University
The Johns Hopkins University, commonly referred to as Johns Hopkins, JHU, or simply Hopkins, is a private research university based in Baltimore, Maryland, United States...
.
The pioneering discoveries and research on water channels by Agre and his colleagues resulted in the presentation of a Nobel Prize
Nobel Prize
The Nobel Prizes are annual international awards bestowed by Scandinavian committees in recognition of cultural and scientific advances. The will of the Swedish chemist Alfred Nobel, the inventor of dynamite, established the prizes in 1895...
in Chemistry to Agre in 2003. In 1999, together with other research teams, Agre reported the first high-resolution images of the three-dimensional structure of an aquaporin, viz. aquaporin-1. Further studies using supercomputer
Supercomputer
A supercomputer is a computer at the frontline of current processing capacity, particularly speed of calculation.Supercomputers are used for highly calculation-intensive tasks such as problems including quantum physics, weather forecasting, climate research, molecular modeling A supercomputer is a...
simulations have identified the pathway of water as it moves through the channel and demonstrated how a pore can allow water to pass without the passage of small solutes.
However the first report of protein mediated water transport through membranes was by Gheorghe Benga
Gheorghe Benga
Gheorghe Benga is a professor in the Department of Cell and Molecular Biology of the University of Medicine and Pharmacy "Iuliu Haţieganu" of Cluj-Napoca, Romania. He is a member of the Romanian Academy....
in 1986. This publication that preceded Agre's first publication on water membrane transport proteins has led to a controversy that Benga's work was adequately recognized by neither Agre nor the Nobel Prize Committee. There is a long history of water pores, starting in 1957. There have been many reviews of the history.
Structure
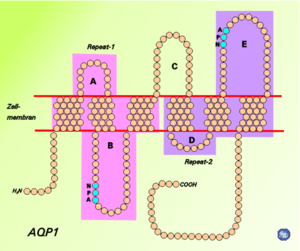
Transmembrane protein
A transmembrane protein is a protein that goes from one side of a membrane through to the other side of the membrane. Many TPs function as gateways or "loading docks" to deny or permit the transport of specific substances across the biological membrane, to get into the cell, or out of the cell as...
α-helices
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
arranged in a right-handed bundle, with the amino and the carboxyl termini located on the cytoplasmic surface of the membrane. The amino and carboxyl halves of the sequence show similarity to each other, in what appears to be a tandem repeat. Some researchers believe that this results from an early evolution event that saw the duplication of the half-size gene. There are also five interhelical loop regions (A – E) that form the extracellular and cytoplasmic vestibules. Loops B and E are hydrophobic loops that contain the highly, although not completely conserved, asparagine
Asparagine
Asparagine is one of the 20 most common natural amino acids on Earth. It has carboxamide as the side-chain's functional group. It is not an essential amino acid...
–proline
Proline
Proline is an α-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG. It is not an essential amino acid, which means that the human body can synthesize it. It is unique among the 20 protein-forming amino acids in that the α-amino group is secondary...
–alanine
Alanine
Alanine is an α-amino acid with the chemical formula CH3CHCOOH. The L-isomer is one of the 20 amino acids encoded by the genetic code. Its codons are GCU, GCC, GCA, and GCG. It is classified as a nonpolar amino acid...
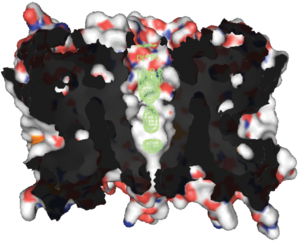
(NPA) motif, which overlap the middle of the lipid bilayer of the membrane forming a 3-D 'hourglass' structure where the water flows through. This overlap forms one of the two well-known channel constriction sites in the peptide, the NPA motif and a second and usually narrower constriction known as 'selectivity filter' or ar/R selectivity filter.
Aquaporins form tetramers in the cell membrane, with each monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
acting as a water channel. The different aquaporins contain differences in their peptide sequence, which allows for the size of the pore in the protein to differ between aquaporins. The resultant size of the pore directly affects what molecules are able to pass through the pore, with small pore sizes only allowing small molecules like water to pass through the pore.
NPA motif
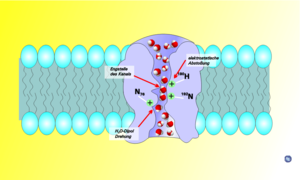
Using computer simulations, it has been suggested that the orientation of the water molecules moving through the channel assures that only water passes between cells, due to the formation of a single line of water molecules. The water molecules move through the narrow channel by orienting themselves in the local electrical field formed by the atoms of the channel wall. Upon entering, the water molecules face with their oxygenOxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
atom down the channel. Midstream, they reverse orientation, facing with the oxygen atom up.
Why this rotation occurs is not entirely clear yet. Some researchers identified an electrostatic field generated by the two aquaporin half-helices HB and HE as the reason for the rotation of water molecules. Others suggested that it is caused by the interaction of hydrogen bonds between the oxygen of the water molecule and the asparagine
Asparagine
Asparagine is one of the 20 most common natural amino acids on Earth. It has carboxamide as the side-chain's functional group. It is not an essential amino acid...
s in the two NPA motifs. Moreover, whether the rotation of water molecules has any biological significance is still being discussed. Early studies speculated that the "bipolar" orientation of water molecules keep them from conducting protons via the Grotthuss mechanism
Grotthuss mechanism
The Grotthuss mechanism is the mechanism by which an 'excess' proton or protonic defect diffuses through the hydrogen bond network of water molecules or other hydrogen-bonded liquids through the formation or cleavage of covalent bonds....
, while still permitting a fast flux of water molecules. More recent studies question this interpretation and emphasize an electrostatic barrier as the reason for proton blockage. In the latter view, the rotation of water molecules is only a side-effect of the electrostatic barrier. At present (2008), the origin of the electrostatic field is a matter of debate. While some studies mainly considered the electric field generated by the protein's half-helices HB and HE, others emphasized desolvation effects as the proton enters the narrow aquaporin pore.
ar/R selectivity filter
In mammals
There are thirteen known types of aquaporins in mammals, and six of these are located in the kidney, but the existence of many more is suspected. The most studied aquaporins are compared in the following table:| Type | Location | Function |
|---|---|---|
| Aquaporin 1 Aquaporin 1 AQP1 is a widely expressed water channel, whose physiological function has been most thoroughly characterized in the kidney.It is found in the basolateral and apical plasma membranes of the proximal tubules, the descending limb of the loop of Henle, and in the descending portion of the vasa... |
|
Water reabsorption |
| Aquaporin 2 Aquaporin 2 AQP2 is found in the apical cell membranes of the kidney's collecting duct principal cells and in intracellular vesicles located throughout the cell.-Regulation:It is the only aquaporin regulated by vasopressin.... |
Kidney The kidneys, organs with several functions, serve essential regulatory roles in most animals, including vertebrates and some invertebrates. They are essential in the urinary system and also serve homeostatic functions such as the regulation of electrolytes, maintenance of acid–base balance, and... (apically)
|
Water reabsorption in response to ADH |
| Aquaporin 3 Aquaporin 3 Aquaporin 3 is found in the basolateral cell membrane of principal collecting duct cells and provide a pathway for water to exit these cells. In kidney, AQP3 gene expression is not regulated by vasopressin . This protein is also a determinant for the GIL blood group system.-External links:* at... |
Kidney The kidneys, organs with several functions, serve essential regulatory roles in most animals, including vertebrates and some invertebrates. They are essential in the urinary system and also serve homeostatic functions such as the regulation of electrolytes, maintenance of acid–base balance, and... (basolaterally)
|
Water reabsorption |
| Aquaporin 4 Aquaporin 4 Aquaporin 4 also known as AQP4 is protein which in humans is encoded by the AQP4 gene. AQP4 belongs to the aquaporin family of integral membrane proteins that conduct water through the cell membrane.- Function :... |
Kidney The kidneys, organs with several functions, serve essential regulatory roles in most animals, including vertebrates and some invertebrates. They are essential in the urinary system and also serve homeostatic functions such as the regulation of electrolytes, maintenance of acid–base balance, and... (basolaterally)
|
Water reabsorption |
In plants
In plants water is taken up from the soil through the roots, where it passes from the cortex into the vascular tissues. There are two routes for water to flow in these tissues, known as the apoplastApoplast
Within a plant, the apoplast is the free diffusional space outside the plasma membrane. It is interrupted by the Casparian strip in roots, air spaces between plant cells and the cuticula of the plant....
ic and symplast
Symplast
The symplast of a plant is the inner side of the plasma membrane in which water can freely diffuse.The plasmodesmata allow the direct flow of small molecules such as sugars, amino acids, and ions between cells...
ic pathways. The presence of aquaporins in the cell membranes seems to serve to facilitate the transcellular symplastic pathway for water transport. When plant roots are exposed to mercuric chloride, which is known to inhibit aquaporins, the flow of water is greatly reduced while the flow of ions is not, supporting the view that there exists a mechanism for water transport independent of the transport of ions; aquaporins.
Aquaporins in plants are separated into five main homologous subfamilies, or groups:
- Plasma membrane Intrinsic Protein (PIP)
- Tonoplast Intrinsic Protein (TIP)
- Nodulin-26 like Intrinsic Protein (NIP)
- Small basic Intrinsic Protein (SIP)
- X Intrinsic Protein (XIP)
These five subfamilies have later been divided into smaller evolutionary subgroups based on their DNA sequence. PIPs cluster into two subgroups, PIP1 and PIP2, whilst TIPs cluster into 5 subgroups, TIP1, TIP2, TIP3, TIP4 and TIP5. Each subgroup is again split up into isoforms e.g. PIP1;1, PIP1;2.
The silencing of plant aquaporins has been linked to poor plant growth and even death of the plant.
The gating
Gating (electrophysiology)
In electrophysiology, the term gating refers to the opening or closing of ion channels.When ion channels are in a 'closed' state, they are impermeable to ions and do not conduct electrical current...
of aquaporins is carried out to stop the flow of water through the pore of the protein. This may be carried out for a number of reasons, for example when the plant contains low amounts of cellular water due to drought. The gating of an aquaporin is carried out by an interaction between a gating mechanism and the aquaporin, which causes a 3D change in the protein so that it blocks the pore and, thus, disallows the flow of water through the pore. In plants, it has been seen that there are at least two forms of aquaporin gating. These are gating by the dephosphorylation of certain serine residues, which has been seen as a response to drought, and the protonation of specific histidine residues in response to flooding. The phosphorylation of an aquaporin has also been linked to the opening and closing of petals in response to temperature.
Clinical significance
If aquaporin could be manipulated, that could potentially solve medical problems such as fluid retention in heart disease and brain edema after stroke, said Agre.There have been two clear examples of diseases identified as resulting from mutations in aquaporins:
- Mutations in the aquaporin-2 geneGeneA gene is a molecular unit of heredity of a living organism. It is a name given to some stretches of DNA and RNA that code for a type of protein or for an RNA chain that has a function in the organism. Living beings depend on genes, as they specify all proteins and functional RNA chains...
cause hereditary nephrogenic diabetes insipidusDiabetes insipidusDiabetes insipidus is a condition characterized by excessive thirst and excretion of large amounts of severely diluted urine, with reduction of fluid intake having no effect on the concentration of the urine. There are several different types of DI, each with a different cause...
in humans. - Mice homozygous for inactivating mutations in the aquaporin-0 gene develop congenital cataracts.
A small number of people have been identified with severe or total deficiency in aquaporin-1. It is interesting to note that they are, in general, healthy, but exhibit a defect in the ability to concentrate solutes in the urine and to conserve water when deprived of drinking water. Mice with targeted deletions in aquaporin-1 also exhibit a deficiency in water conservation due to an inability to concentrate solutes in the kidney medulla by countercurrent multiplication
Countercurrent multiplication
A countercurrent multiplier system is a mechanism that expends energy to create a concentration gradient.It is found widely in nature and especially in mamalian organs...
.
In addition to its role in genetically determined nephrogenic diabetes insipidus, aquaporins also play a key role in acquired forms of nephrogenic diabetes insipidus
Nephrogenic diabetes insipidus
Nephrogenic diabetes insipidus is a form of diabetes insipidus due primarily to pathology of the kidney. This is in contrast to central/neurogenic diabetes insipidus, which is caused by insufficient levels of antidiuretic hormone /Argenine Vasopressin...
(disorders that cause increased urine production). Acquired nephrogenic diabetes insipidus can result from impaired regulation of aquaporin-2 due to administration of lithium salts (as a treatment for bipolar disorder
Bipolar disorder
Bipolar disorder or bipolar affective disorder, historically known as manic–depressive disorder, is a psychiatric diagnosis that describes a category of mood disorders defined by the presence of one or more episodes of abnormally elevated energy levels, cognition, and mood with or without one or...
), low potassium concentrations in the blood (hypokalemia), high calcium concentrations in the blood (hypercalcemia), or a chronically high intake of water beyond the normal requirements (e.g., due to excessive habitual intake of bottled water or coffee).
It has been found that autoimmune reactions against aquaporin 4
Aquaporin 4
Aquaporin 4 also known as AQP4 is protein which in humans is encoded by the AQP4 gene. AQP4 belongs to the aquaporin family of integral membrane proteins that conduct water through the cell membrane.- Function :...
produce Devic's disease
Devic's disease
Neuromyelitis optica , also known as Devic's disease or Devic's syndrome, is an autoimmune, inflammatory disorder in which a person's own immune system attacks the optic nerves and spinal cord. This produces an inflammation of the optic nerve and the spinal cord...
.
External links
- Animation (MPEG file at nobel.se)

