
Applied spectroscopy
Encyclopedia
Applied spectroscopy is the application of various spectroscopic
methods
for detection and identification of different element
s/compound
s in solving problems in the fields of forensics
, medicine
, oil industry, atmospheric chemistry
, pharmacology
, etc.
UV spectroscopy is used where strong absorption of ultra-violet radiation occurs in a substance. Such groups are known as chromophores and include aromatic groups, conjugated system
of bonds, carbonyl groups and so on. NMR spectroscopy
detects hydrogen atoms in specific environments, and complements both IR and UV spectroscopy. The use of Raman spectroscopy
is growing for more specialist applications.
There are also derivative methods such as infrared microscopy, which allows very small areas to be analysed in an optical microscope
.
One method of elemental analysis
that is important in forensic analysis is EDX
performed in the environmental scanning electron microscope, or ESEM
. The method involves analysis of back-scattered X-rays from the sample as a result of interaction with the electron beam. Automated SEM-EDS analysis is further used in a range of Automated Mineralogy
quantitative mineral
, identification and textural mapping, such as the QEMSCAN
and MLA
solutions.
FTIR: Three types of samples can be analyzed, a solution (KBr), a powder, or a film. A solid film is the easiest and most straight forward sample type to test.
mechanisms can be followed using infra-red spectroscopy, such as UV degradation
and oxidation, among many other failure modes.

suffers severe cracking in sunlight
unless anti-oxidants are added. The point of attack occurs at the tertiary carbon atom present in every repeat unit, causing oxidation and finally chain breakage. Polyethylene
is also susceptible to UV degradation, especially those variants that are branched polymers such as LDPE. The branch points are tertiary carbon atoms, so polymer degradation
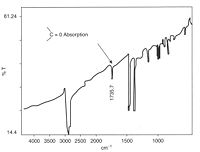
starts there and results in chain cleavage, and embrittlement. In the example shown at left, carbonyl groups were readily detected by IR spectroscopy from a cast thin film. The product was a road cone that had cracked in service, and many similar cones also failed because an anti-UV additive had not been used.
 Polymers are susceptible to attack by atmospheric oxygen
Polymers are susceptible to attack by atmospheric oxygen
, especially at elevated temperatures encountered during processing to shape. Many process methods such as extrusion
and injection moulding
involve pumping molten polymer into tools, and the high temperatures needed for melting may result in oxidation unless precautions are taken. For example, a forearm crutch
suddenly snapped and the user was severely injured in the resulting fall. The crutch had fractured across a polypropylene
insert within the aluminium tube of the device, and infra-red spectroscopy of the material showed that it had oxidised, possible as a result of poor moulding.
Oxidation is usually relatively easy to detect, owing to the strong absorption by the carbonyl group in the spectrum of polyolefins. Polypropylene
has a relatively simple spectrum, with few peaks at the carbonyl position (like polyethylene
). Oxidation tends to start at tertiary carbon atoms because free radicals here at more stable, so last longer and are attacked by oxygen
. The carbonyl group can be further oxidised to break the chain, so weakening the material by lowering the molecular weight, and cracks start to grow in the regions affected.
when one molecule of the gas reacts with the double bond:
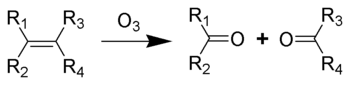 The immediate result is formation of an ozonide
The immediate result is formation of an ozonide
, which then decomposes rapidly so that the double bond is cleaved. This is the critical step in chain breakage when polymers are attacked. The strength of polymers depends on the chain molecular weight or degree of polymerization
: The higher the chain length the greater the mechanical strength (such as tensile strength
). By cleaving the chain, the molecular weight drops rapidly and there comes a point when it has little strength whatsoever, and a crack forms. Further attack occurs in the freshly exposed crack surfaces and the crack grows steadily until it completes a circuit and the product separates or fails. In the case of a seal or a tube, failure occurs when the wall of the device is penetrated.
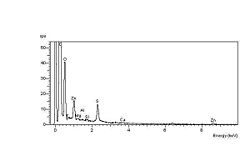
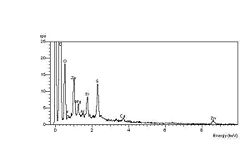 The carbonyl end groups that are formed are usually aldehydes or ketone
The carbonyl end groups that are formed are usually aldehydes or ketone
s, which can oxidise further to carboxylic acid
s. The net result is a high concentration of elemental oxygen on the crack surfaces, which can be detected using Energy-dispersive X-ray spectroscopy
in the environmental SEM, or ESEM
. The spectrum at left shows the high-oxygen peak compared with a constant sulphur peak. The spectrum at right shows the unaffected elastomer surface spectrum, with a relatively low-oxygen peak compared with the sulphur peak. The spectra were obtained during an investigation into ozone cracking
of diaphragm seal
s in a semi-conductor fabrication factory.
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
methods
Methodology
Methodology is generally a guideline for solving a problem, with specificcomponents such as phases, tasks, methods, techniques and tools . It can be defined also as follows:...
for detection and identification of different element
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
s/compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s in solving problems in the fields of forensics
Forensics
Forensic science is the application of a broad spectrum of sciences to answer questions of interest to a legal system. This may be in relation to a crime or a civil action...
, medicine
Medicine
Medicine is the science and art of healing. It encompasses a variety of health care practices evolved to maintain and restore health by the prevention and treatment of illness....
, oil industry, atmospheric chemistry
Atmospheric chemistry
Atmospheric chemistry is a branch of atmospheric science in which the chemistry of the Earth's atmosphere and that of other planets is studied. It is a multidisciplinary field of research and draws on environmental chemistry, physics, meteorology, computer modeling, oceanography, geology and...
, pharmacology
Pharmacology
Pharmacology is the branch of medicine and biology concerned with the study of drug action. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function...
, etc.
Spectroscopic methods
A common spectroscopic method for analysis is FTIR spectroscopy, where chemical bonds can be detected through their characteristic infra-red absorption frequencies or wavelengths. These absorption characteristics make infrared analysers an invaluable tool in geoscience, environmental science and atmospheric science. For instance, atmospheric gas monitoring has been facilitated by the development of commercially available gas analysers which can distinguish between carbon dioxide, methane, carbon monoxide, oxygen and nitric oxide.UV spectroscopy is used where strong absorption of ultra-violet radiation occurs in a substance. Such groups are known as chromophores and include aromatic groups, conjugated system
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
of bonds, carbonyl groups and so on. NMR spectroscopy
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
detects hydrogen atoms in specific environments, and complements both IR and UV spectroscopy. The use of Raman spectroscopy
Raman spectroscopy
Raman spectroscopy is a spectroscopic technique used to study vibrational, rotational, and other low-frequency modes in a system.It relies on inelastic scattering, or Raman scattering, of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range...
is growing for more specialist applications.
There are also derivative methods such as infrared microscopy, which allows very small areas to be analysed in an optical microscope
Optical microscope
The optical microscope, often referred to as the "light microscope", is a type of microscope which uses visible light and a system of lenses to magnify images of small samples. Optical microscopes are the oldest design of microscope and were possibly designed in their present compound form in the...
.
One method of elemental analysis
Elemental analysis
Percent Composition is a process where a sample of some material is analyzed for its elemental and sometimes isotopic composition. Elemental analysis can be qualitative , and it can be quantitative...
that is important in forensic analysis is EDX
Energy-dispersive X-ray spectroscopy
Energy-dispersive X-ray spectroscopy is an analytical technique used for the elemental analysis or chemical characterization of a sample. It relies on the investigation of an interaction of a some source of X-ray excitation and a sample...
performed in the environmental scanning electron microscope, or ESEM
ESEM
The environmental scanning electron microscope or ESEM is a scanning electron microscope that allows for the option of collecting electron micrographs of specimens that are "wet," uncoated, or both by allowing for a gaseous environment in the specimen chamber...
. The method involves analysis of back-scattered X-rays from the sample as a result of interaction with the electron beam. Automated SEM-EDS analysis is further used in a range of Automated Mineralogy
Automated mineralogy
Automated mineralogy is a generic term describing a range of analytical solutions, areas of commercial enterprise, and a growing field of scientific research and engineering applications involving largely automated and quantitative analysis of minerals, rocks and man-made...
quantitative mineral
Mineral
A mineral is a naturally occurring solid chemical substance formed through biogeochemical processes, having characteristic chemical composition, highly ordered atomic structure, and specific physical properties. By comparison, a rock is an aggregate of minerals and/or mineraloids and does not...
, identification and textural mapping, such as the QEMSCAN
QEMSCAN
QEMSCAN is the name for an integrated automated mineralogy and petrography solution providing quantitative analysis of minerals, rocks and man-made materials. QEMSCAN is an abbreviation standing for Quantitative Evaluation of Minerals by SCANning electron microscopy, and a registered trademark...
and MLA
MLA
- Organizations :* Modern Language Association, United States of America** The MLA Style Manual, its works cited page guidelines* Massachusetts Library Association* Medical Library Association, United States...
solutions.
Sample preparation
In all three spectroscopic methods, the sample usually needs to be present in solution, which may present problems during forensic examination because it necessarily involves sampling solid from the object to be examined.FTIR: Three types of samples can be analyzed, a solution (KBr), a powder, or a film. A solid film is the easiest and most straight forward sample type to test.
Analysis of polymers
Many polymer degradationPolymer degradation
Polymer degradation is a change in the properties—tensile strength, colour, shape, etc.—of a polymer or polymer-based product under the influence of one or more environmental factors such as heat, light or chemicals such as acids, alkalis and some salts...
mechanisms can be followed using infra-red spectroscopy, such as UV degradation
UV degradation
Many natural and synthetic polymers are attacked by ultra-violet radiation and products made using these materials may crack or disintegrate . The problem is known as UV degradation, and is a common problem in products exposed to sunlight...
and oxidation, among many other failure modes.

UV degradation
Many polymers are attacked by UV radiation at vulnerable points in their chain structures. Thus, polypropylenePolypropylene
Polypropylene , also known as polypropene, is a thermoplastic polymer used in a wide variety of applications including packaging, textiles , stationery, plastic parts and reusable containers of various types, laboratory equipment, loudspeakers, automotive components, and polymer banknotes...
suffers severe cracking in sunlight
Sunlight
Sunlight, in the broad sense, is the total frequency spectrum of electromagnetic radiation given off by the Sun. On Earth, sunlight is filtered through the Earth's atmosphere, and solar radiation is obvious as daylight when the Sun is above the horizon.When the direct solar radiation is not blocked...
unless anti-oxidants are added. The point of attack occurs at the tertiary carbon atom present in every repeat unit, causing oxidation and finally chain breakage. Polyethylene
Polyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
is also susceptible to UV degradation, especially those variants that are branched polymers such as LDPE. The branch points are tertiary carbon atoms, so polymer degradation
Polymer degradation
Polymer degradation is a change in the properties—tensile strength, colour, shape, etc.—of a polymer or polymer-based product under the influence of one or more environmental factors such as heat, light or chemicals such as acids, alkalis and some salts...
starts there and results in chain cleavage, and embrittlement. In the example shown at left, carbonyl groups were readily detected by IR spectroscopy from a cast thin film. The product was a road cone that had cracked in service, and many similar cones also failed because an anti-UV additive had not been used.
Oxidation

Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, especially at elevated temperatures encountered during processing to shape. Many process methods such as extrusion
Extrusion
Extrusion is a process used to create objects of a fixed cross-sectional profile. A material is pushed or drawn through a die of the desired cross-section...
and injection moulding
Injection moulding
Injection molding is a manufacturing process for producing parts from both thermoplastic and thermosetting plastic materials. Material is fed into a heated barrel, mixed, and forced into a mold cavity where it cools and hardens to the configuration of the cavity...
involve pumping molten polymer into tools, and the high temperatures needed for melting may result in oxidation unless precautions are taken. For example, a forearm crutch
Crutch
Crutches are mobility aids used to counter a mobility impairment or an injury that limits walking ability.- Types :There are several different types of crutches:...
suddenly snapped and the user was severely injured in the resulting fall. The crutch had fractured across a polypropylene
Polypropylene
Polypropylene , also known as polypropene, is a thermoplastic polymer used in a wide variety of applications including packaging, textiles , stationery, plastic parts and reusable containers of various types, laboratory equipment, loudspeakers, automotive components, and polymer banknotes...
insert within the aluminium tube of the device, and infra-red spectroscopy of the material showed that it had oxidised, possible as a result of poor moulding.
Oxidation is usually relatively easy to detect, owing to the strong absorption by the carbonyl group in the spectrum of polyolefins. Polypropylene
Polypropylene
Polypropylene , also known as polypropene, is a thermoplastic polymer used in a wide variety of applications including packaging, textiles , stationery, plastic parts and reusable containers of various types, laboratory equipment, loudspeakers, automotive components, and polymer banknotes...
has a relatively simple spectrum, with few peaks at the carbonyl position (like polyethylene
Polyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
). Oxidation tends to start at tertiary carbon atoms because free radicals here at more stable, so last longer and are attacked by oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
. The carbonyl group can be further oxidised to break the chain, so weakening the material by lowering the molecular weight, and cracks start to grow in the regions affected.
Ozonolysis
The reaction occurring between double bonds and ozone is known as ozonolysisOzonolysis
Ozonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon–carbon bond has been replaced by a double bond to oxygen...
when one molecule of the gas reacts with the double bond:

Ozonide
Ozonide is an unstable, reactive polyatomic anion O3−, derived from ozone, or an organic compound similar to organic peroxide formed by a reaction of ozone with an unsaturated compound.-Inorganic ozonides:...
, which then decomposes rapidly so that the double bond is cleaved. This is the critical step in chain breakage when polymers are attacked. The strength of polymers depends on the chain molecular weight or degree of polymerization
Degree of polymerization
The degree of polymerization, or DP, is usually defined as the number of monomeric units in a macromolecule or polymer or oligomer molecule.For a homopolymer, there is only one type of monomeric unit andthe number-average degree of polymerization is given by...
: The higher the chain length the greater the mechanical strength (such as tensile strength
Tensile strength
Ultimate tensile strength , often shortened to tensile strength or ultimate strength, is the maximum stress that a material can withstand while being stretched or pulled before necking, which is when the specimen's cross-section starts to significantly contract...
). By cleaving the chain, the molecular weight drops rapidly and there comes a point when it has little strength whatsoever, and a crack forms. Further attack occurs in the freshly exposed crack surfaces and the crack grows steadily until it completes a circuit and the product separates or fails. In the case of a seal or a tube, failure occurs when the wall of the device is penetrated.


Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s, which can oxidise further to carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s. The net result is a high concentration of elemental oxygen on the crack surfaces, which can be detected using Energy-dispersive X-ray spectroscopy
Energy-dispersive X-ray spectroscopy
Energy-dispersive X-ray spectroscopy is an analytical technique used for the elemental analysis or chemical characterization of a sample. It relies on the investigation of an interaction of a some source of X-ray excitation and a sample...
in the environmental SEM, or ESEM
ESEM
The environmental scanning electron microscope or ESEM is a scanning electron microscope that allows for the option of collecting electron micrographs of specimens that are "wet," uncoated, or both by allowing for a gaseous environment in the specimen chamber...
. The spectrum at left shows the high-oxygen peak compared with a constant sulphur peak. The spectrum at right shows the unaffected elastomer surface spectrum, with a relatively low-oxygen peak compared with the sulphur peak. The spectra were obtained during an investigation into ozone cracking
Ozone cracking
Cracks can be formed in many different elastomers by ozone attack, and the characteristic form of attack of vulnerable rubbers is known as ozone cracking...
of diaphragm seal
Diaphragm seal
A diaphragm seal is a flexible membrane that seals and isolates an enclosure. The flexible nature of this seal allows pressure effects to cross the barrier but not the material being contained....
s in a semi-conductor fabrication factory.
See also
- Absorption spectroscopyAbsorption spectroscopyAbsorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a...
- Infrared spectroscopy correlation tableInfrared spectroscopy correlation tableIn physical and analytical chemistry, infrared spectroscopy is a technique used to identify chemical compounds based on how infrared radiation is absorbed by the compounds' chemical bonds. This article is an IR spectroscopy correlation table that lists some general absorption peaks for common...
- Infrared spectroscopyInfrared spectroscopyInfrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
- Forensic chemistryForensic chemistryForensic chemistry is the application of chemistry to law enforcement or the failure of products or processes. Many different analytical methods may be used to reveal what chemical changes occurred during an incident, and so help reconstruct the sequence of events...
- Forensic engineeringForensic engineeringForensic engineering is the investigation of materials, products, structures or components that fail or do not operate or function as intended, causing personal injury or damage to property. The consequences of failure are dealt with by the law of product liability. The field also deals with...
- Forensic polymer engineeringForensic polymer engineeringThe study of failure in polymeric products is called forensic polymer engineering. The topic includes the fracture of plastic products, or any other reason why such a product fails in service, or fails to meet its specification...
- Polymer degradationPolymer degradationPolymer degradation is a change in the properties—tensile strength, colour, shape, etc.—of a polymer or polymer-based product under the influence of one or more environmental factors such as heat, light or chemicals such as acids, alkalis and some salts...
- Polymer engineeringPolymer engineeringPolymer engineering is generally an engineering field that designs, analyses, and/or modifies polymer materials. Polymer engineering covers aspects of petrochemical industry, polymerization, structure and characterization of polymers, properties of polymers, compounding and processing of polymers...
- SpectroscopySpectroscopySpectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
- Society for Applied SpectroscopySociety for Applied SpectroscopyThe Society for Applied Spectroscopy is an organization promoting research and education in the fields of spectroscopy, optics, and analytical chemistry. Founded in 1958, it is currently headquartered in Frederick, MD...
- Slope spectroscopySlope SpectroscopySlope spectroscopy is a spectroscopic technique that is used to quantify concentrations of various compounds, proteins and antibodies in which the operating path length is varied and the absorbance is measured.- Equations :...

