
Slope Spectroscopy
Encyclopedia
Slope spectroscopy is a spectroscopic technique that is used to quantify concentrations of various compounds, proteins and antibodies in which the operating path length
is varied and the absorbance is measured.
states that there is a logarithmic
dependence between the transmission
(or transmissivity), T, of light through a substance and the product of the absorption coefficient of the substance, α, and the distance the light
travels through the material (i.e. the path length), ℓ. The absorption coefficient can, in turn, be written as a product of either a molar absorptivity
of the absorber, ε, and the concentration c of absorbing species in the material, or an absorption cross section
, σ, and the (number) density N of absorbers. (see Beer Lambert Law link for full derivation)

Slope spectroscopy takes advantage of Beer-Lambert law to determine concentrations of various solutions. By knowing the molar absorbtivity of the material and varying the path length, absorption can be plotted as a function of path length. See sample plot to the right:
By taking a linear regression
of the linear plot above an expression relating Absorbance, A, slope, m, pathlength and concentration can be derived.
A linear equation of two variables can be derived,

by equating in terms of units we get,

Since the slope of the line is in units of Abs/Pathlength, slope can be expressed as,
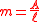
by inserting into Beer’s Law we get,

This is the slope spectroscopy equation.
Slope spectroscopy is typically used when highly reproducible data is a necessity. This can be in the fields of medicine
, biotechnology
, pharmacology
, and drug discovery
. It is particularly useful in the protein purification
stage of biotechnology where accurate concentrations of various proteins are required or in crystallography
.
Determining the relative ratio of protein to DNA is common practice and can be calculated by finding the slope at the corresponding absorption peaks and taking their ratio. This method is used to find the purity of a sample containing these two types of molecule.
or spectroscopy
in general a 1 cm pathlength cuvette
is used to measure samples. The cuvette is filled with sample, light is passed through the sample and intensity readings are taken. The slope spectroscopy technique can be applied using the same methods as in absorption spectroscopy
. With the advent of accurate linear stages, the slope spectroscopy technique is easily applied experimentally.
Other experimental methods include using ratios of slopes to build extinction coefficient spectra. This is possible because application of slope spectroscopy allows the scientist to keep concentration levels constant and vary path lengths.
This shows that the absorbance values on the plot are offset by an equal amount and the slope of the two plots are equal. Thus, the concentration calculated from the two plots is equal. Other scalar components that contribute to the absorbance of a given sample like contaminants on the cuvette or a different cuvette material also are averaged out during the slope measurement.
Path length
In chemistry, the path length is defined as the distance that light travels through a sample in an analytical cell. Typically, a sample cell is made of quartz, glass, or a plastic rhombic cuvette with a volume typically ranging from 0.1 mL to 10 mL or larger used in a spectrophotometer. For the...
is varied and the absorbance is measured.
Equations
The Beer-Lambert lawBeer-Lambert law
In optics, the Beer–Lambert law, also known as Beer's law or the Lambert–Beer law or the Beer–Lambert–Bouguer law relates the absorption of light to the properties of the material through which the light is travelling.-Equations:The law states that there is a logarithmic dependence between the...
states that there is a logarithmic
Logarithmic
Logarithmic can refer to:* Logarithm, a transcendental function in mathematics* Logarithmic scale, the use of the logarithmic function to describe measurements* Logarithmic growth* Logarithmic distribution, a discrete probability distribution...
dependence between the transmission
Transmission coefficient
The transmission coefficient is used in physics and electrical engineering when wave propagation in a medium containing discontinuities is considered...
(or transmissivity), T, of light through a substance and the product of the absorption coefficient of the substance, α, and the distance the light
Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
travels through the material (i.e. the path length), ℓ. The absorption coefficient can, in turn, be written as a product of either a molar absorptivity
Molar absorptivity
The molar absorption coefficient, molar extinction coefficient, or molar absorptivity, is a measurement of how strongly a chemical species absorbs light at a given wavelength...
of the absorber, ε, and the concentration c of absorbing species in the material, or an absorption cross section
Absorption cross section
Absorption cross section is a measure for the probability of an absorption process. More generally, the term cross section is used in physics to quantify the probability of a certain particle-particle interaction, e.g., scattering, electromagnetic absorption, etc...
, σ, and the (number) density N of absorbers. (see Beer Lambert Law link for full derivation)

Slope spectroscopy takes advantage of Beer-Lambert law to determine concentrations of various solutions. By knowing the molar absorbtivity of the material and varying the path length, absorption can be plotted as a function of path length. See sample plot to the right:
By taking a linear regression
Linear regression
In statistics, linear regression is an approach to modeling the relationship between a scalar variable y and one or more explanatory variables denoted X. The case of one explanatory variable is called simple regression...
of the linear plot above an expression relating Absorbance, A, slope, m, pathlength and concentration can be derived.
A linear equation of two variables can be derived,

by equating in terms of units we get,

Since the slope of the line is in units of Abs/Pathlength, slope can be expressed as,
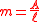
by inserting into Beer’s Law we get,

This is the slope spectroscopy equation.
Applications
The slope spectroscopy technique can be applied in any situation where Beer’s law can be applied. It provides an analytical method that averages out minor variations in sample preparation consistency. It also provides a means to calculate concentrations without calibrations curves or serial dilution of samples.Slope spectroscopy is typically used when highly reproducible data is a necessity. This can be in the fields of medicine
Medicine
Medicine is the science and art of healing. It encompasses a variety of health care practices evolved to maintain and restore health by the prevention and treatment of illness....
, biotechnology
Biotechnology
Biotechnology is a field of applied biology that involves the use of living organisms and bioprocesses in engineering, technology, medicine and other fields requiring bioproducts. Biotechnology also utilizes these products for manufacturing purpose...
, pharmacology
Pharmacology
Pharmacology is the branch of medicine and biology concerned with the study of drug action. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function...
, and drug discovery
Drug discovery
In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which drugs are discovered or designed.In the past most drugs have been discovered either by identifying the active ingredient from traditional remedies or by serendipitous discovery...
. It is particularly useful in the protein purification
Protein purification
Protein purification is a series of processes intended to isolate a single type of protein from a complex mixture. Protein purification is vital for the characterization of the function, structure and interactions of the protein of interest. The starting material is usually a biological tissue or...
stage of biotechnology where accurate concentrations of various proteins are required or in crystallography
Crystallography
Crystallography is the experimental science of the arrangement of atoms in solids. The word "crystallography" derives from the Greek words crystallon = cold drop / frozen drop, with its meaning extending to all solids with some degree of transparency, and grapho = write.Before the development of...
.
Determining the relative ratio of protein to DNA is common practice and can be calculated by finding the slope at the corresponding absorption peaks and taking their ratio. This method is used to find the purity of a sample containing these two types of molecule.
Experimental methods
In ultraviolet-visible spectroscopyUltraviolet-visible spectroscopy
Ultraviolet-visible spectroscopy or ultraviolet-visible spectrophotometry refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent ranges...
or spectroscopy
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
in general a 1 cm pathlength cuvette
Cuvette
A cuvette is a small tube of circular or square cross section, sealed at one end, made of plastic, glass, or fused quartz and designed to hold samples for spectroscopic experiments. The best cuvettes are as clear as possible, without impurities that might affect a spectroscopic reading...
is used to measure samples. The cuvette is filled with sample, light is passed through the sample and intensity readings are taken. The slope spectroscopy technique can be applied using the same methods as in absorption spectroscopy
Absorption spectroscopy
Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a...
. With the advent of accurate linear stages, the slope spectroscopy technique is easily applied experimentally.
Other experimental methods include using ratios of slopes to build extinction coefficient spectra. This is possible because application of slope spectroscopy allows the scientist to keep concentration levels constant and vary path lengths.
Background subtraction
The slope spectroscopy technique uses a determined slope to calculate concentration. As stated above this is a product of the molar absorbtivity and the concentration. Since the actual absorbance value is taken at many data points at equal intervals, background subtraction is generally unnecessary. The image on the right is a linear plot showing both the background corrected data and the raw data.This shows that the absorbance values on the plot are offset by an equal amount and the slope of the two plots are equal. Thus, the concentration calculated from the two plots is equal. Other scalar components that contribute to the absorbance of a given sample like contaminants on the cuvette or a different cuvette material also are averaged out during the slope measurement.
See also
- Applied spectroscopyApplied spectroscopyApplied spectroscopy is the application of various spectroscopic methods for detection and identification of different elements/compounds in solving problems in the fields of forensics, medicine, oil industry, atmospheric chemistry, pharmacology, etc....
- Absorption spectroscopyAbsorption spectroscopyAbsorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a...
- Quantification of nucleic acidsQuantification of nucleic acidsIn molecular biology, quantitation of nucleic acids is commonly performed to determine the average concentrations of DNA or RNA present in a mixture, as well as their purity. Reactions that use nucleic acids often require particular amounts and purity for optimum performance...
- Polymer degradationPolymer degradationPolymer degradation is a change in the properties—tensile strength, colour, shape, etc.—of a polymer or polymer-based product under the influence of one or more environmental factors such as heat, light or chemicals such as acids, alkalis and some salts...
- ChromatographyChromatographyChromatography is the collective term for a set of laboratory techniques for the separation of mixtures....
- Forensic polymer engineeringForensic polymer engineeringThe study of failure in polymeric products is called forensic polymer engineering. The topic includes the fracture of plastic products, or any other reason why such a product fails in service, or fails to meet its specification...
- Society for Applied SpectroscopySociety for Applied SpectroscopyThe Society for Applied Spectroscopy is an organization promoting research and education in the fields of spectroscopy, optics, and analytical chemistry. Founded in 1958, it is currently headquartered in Frederick, MD...

