
Ozonide
Encyclopedia
Ozonide is an unstable, reactive polyatomic
anion O3−, derived from ozone
, or an organic compound similar to organic peroxide
formed by a reaction of ozone with an unsaturated compound
.
s containing the reactive O3− anion. The anion has the V shape of the ozone molecule.
Inorganic ozonides are formed by burning potassium
or heavier alkali metal
s in ozone
, or by treating the alkali metal hydroxide with ozone; if potassium is left undisturbed in air for years it accumulates a covering of superoxide and ozonide. They are very sensitive explosives that have to be handled at low temperatures in an atmosphere consisting of an inert gas
. Lithium and sodium ozonide are extremely unstable and must be prepared by low-temperature ion exchange starting from CsO3. Sodium ozonide, , which is prone to decompose into NaOH and
, was previously thought to be impossible to obtain in pure form. However, with the help of cryptand
s and methylamine
, pure may be obtained as red crystals isostructural to
.
Inorganic ozonides are being investigated as promising sources of oxygen
in chemical oxygen generator
s.
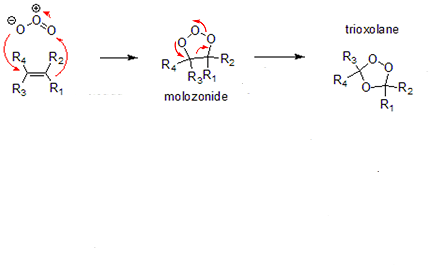
s formed by addition reactions of ozone and unsaturated compound
s. They are intermediates
in ozonolysis
and have a trioxolane ring structure with a five-membered C-O-O-C-O ring. They usually appear in the form of foul-smelling oily liquids, and rapidly decompose in the presence of water to carbonyl
compounds: aldehyde
s, ketone
s, peroxide
s. Due to their instability, they are rarely isolated during the course of the ozonolysis reaction sequence.
Polyatomic ion
A polyatomic ion, also known as a molecular ion, is a charged species composed of two or more atoms covalently bonded or of a metal complex that can be considered as acting as a single unit in the context of acid and base chemistry or in the formation of salts. The prefix "poly-" means "many," in...
anion O3−, derived from ozone
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
, or an organic compound similar to organic peroxide
Organic peroxide
Organic peroxides are organic compounds containing the peroxide functional group . If the R' is hydrogen, the compound is called an organic hydroperoxide. Peresters have general structure RCOOR. The O-O bond easily breaks and forms free radicals of the form RO·...
formed by a reaction of ozone with an unsaturated compound
Unsaturated compound
In organic chemistry, a saturated compound is a chemical compound that has of a chain of carbon atoms linked together by single bonds and has hydrogen atoms filling all of the other bonding orbitals of the carbon atoms. Alkanes are an example of saturated compounds...
.
Inorganic ozonides
Inorganic ozonides are dark red ionic compoundIonic compound
In chemistry, an ionic compound is a chemical compound in which ions are held together in a lattice structure by ionic bonds. Usually, the positively charged portion consists of metal cations and the negatively charged portion is an anion or polyatomic ion. Ions in ionic compounds are held together...
s containing the reactive O3− anion. The anion has the V shape of the ozone molecule.
Inorganic ozonides are formed by burning potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
or heavier alkali metal
Alkali metal
The alkali metals are a series of chemical elements in the periodic table. In the modern IUPAC nomenclature, the alkali metals comprise the group 1 elements, along with hydrogen. The alkali metals are lithium , sodium , potassium , rubidium , caesium , and francium...
s in ozone
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
, or by treating the alkali metal hydroxide with ozone; if potassium is left undisturbed in air for years it accumulates a covering of superoxide and ozonide. They are very sensitive explosives that have to be handled at low temperatures in an atmosphere consisting of an inert gas
Inert gas
An inert gas is a non-reactive gas used during chemical synthesis, chemical analysis, or preservation of reactive materials. Inert gases are selected for specific settings for which they are functionally inert since the cost of the gas and the cost of purifying the gas are usually a consideration...
. Lithium and sodium ozonide are extremely unstable and must be prepared by low-temperature ion exchange starting from CsO3. Sodium ozonide, , which is prone to decompose into NaOH and
Sodium superoxide
Sodium superoxide is the inorganic compound with the formula NaO2. This yellow-orange solid is a salt of the superoxide anion. It is an intermediate in the oxidation of sodium by oxygen....
, was previously thought to be impossible to obtain in pure form. However, with the help of cryptand
Cryptand
Cryptands are a family of synthetic bi- and polycyclic multidentate ligands for a variety of cations. The Nobel Prize for Chemistry in 1987 was given to Donald J. Cram, Jean-Marie Lehn, and Charles J. Pedersen for their efforts in discovering and determining uses of cryptands and crown ethers,...
s and methylamine
Methylamine
Methylamine is the organic compound with a formula of CH3NH2. This colourless gas is a derivative of ammonia, but with one H atom replaced by a methyl group. It is the simplest primary amine. It is sold as a solution in methanol, ethanol, THF, and water, or as the anhydrous gas in pressurized...
, pure may be obtained as red crystals isostructural to
Sodium nitrite
Sodium nitrite is the inorganic compound with the chemical formula NaNO2. It is a white to slight yellowish crystalline powder that is very soluble in water and is hygroscopic...
.
Inorganic ozonides are being investigated as promising sources of oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
in chemical oxygen generator
Chemical oxygen generator
A chemical oxygen generator is a device releasing oxygen created by a chemical reaction. The oxygen source is usually an inorganic superoxide, chlorate, or perchlorate. A promising group of oxygen sources are ozonides. The generators are usually ignited mechanically, by a firing pin, and the...
s.
Organic ozonides
Organic ozonides are more explosive cousins of the organic peroxideOrganic peroxide
Organic peroxides are organic compounds containing the peroxide functional group . If the R' is hydrogen, the compound is called an organic hydroperoxide. Peresters have general structure RCOOR. The O-O bond easily breaks and forms free radicals of the form RO·...
s formed by addition reactions of ozone and unsaturated compound
Unsaturated compound
In organic chemistry, a saturated compound is a chemical compound that has of a chain of carbon atoms linked together by single bonds and has hydrogen atoms filling all of the other bonding orbitals of the carbon atoms. Alkanes are an example of saturated compounds...
s. They are intermediates
Reactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
in ozonolysis
Ozonolysis
Ozonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon–carbon bond has been replaced by a double bond to oxygen...
and have a trioxolane ring structure with a five-membered C-O-O-C-O ring. They usually appear in the form of foul-smelling oily liquids, and rapidly decompose in the presence of water to carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
compounds: aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s, ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s, peroxide
Peroxide
A peroxide is a compound containing an oxygen–oxygen single bond or the peroxide anion .The O−O group is called the peroxide group or peroxo group. In contrast to oxide ions, the oxygen atoms in the peroxide ion have an oxidation state of −1.The simplest stable peroxide is hydrogen peroxide...
s. Due to their instability, they are rarely isolated during the course of the ozonolysis reaction sequence.

See also
- ozonolysisOzonolysisOzonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon–carbon bond has been replaced by a double bond to oxygen...
- ozoneOzoneOzone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
- ozone crackingOzone crackingCracks can be formed in many different elastomers by ozone attack, and the characteristic form of attack of vulnerable rubbers is known as ozone cracking...
- superoxideSuperoxideA superoxide, also known by the obsolete name hyperoxide, is a compound that possesses the superoxide anion with the chemical formula O2−. The systematic name of the anion is dioxide. It is important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature...
, O2− - peroxidePeroxideA peroxide is a compound containing an oxygen–oxygen single bond or the peroxide anion .The O−O group is called the peroxide group or peroxo group. In contrast to oxide ions, the oxygen atoms in the peroxide ion have an oxidation state of −1.The simplest stable peroxide is hydrogen peroxide...
, O22− - oxideOxideAn oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
, O2− - dioxygenylDioxygenylThe dioxygenyl ion, O2+, is a rarely encountered oxycation in which both oxygen atoms have a formal oxidation state of +½. It is formally derived from oxygen by the removal of an electron:...
, O2+

