Alpha 2-macroglobulin
Encyclopedia
alpha-2-Macroglobulin, also known as α2-macroglobulin and abbreviated as α2M and A2M, is a large plasma protein found in the blood
. It is produced by the liver
, and is a major component of the alpha-2 band in protein electrophoresis
.
Alpha 2-Macroglobulin is the largest major nonimmunoglobulin protein in plasma. The alpha 2-macroglobulin molecule is synthesized mainly in liver, but also locally by macrophages, fibroblasts, and adrenocortical cells.
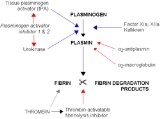
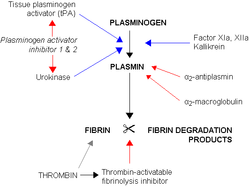
Alpha 2 macroglobulin acts as an antiprotease and is able to inactivate an enormous variety of proteinases. It functions as an inhibitor of fibrinolysis by inhibiting plasmin and kallikrein. It functions as an inhibitor of coagulation by inhibiting thrombin. Alpha 2-macroglobulin may act as a carrier protein because it also binds to numerous growth factors and cytokines, such as platelet-derived growth factor, basic fibroblast growth factor, TGF-β, insulin, and IL-1β.
No specific deficiency with associated disease has been recognized, and no disease state is attributed to low concentrations of Alpha 2 macroglobulin.
The concentration of alpha 2 macroglobulin rises 10-fold or more in the nephrotic syndrome when other lower molecular weight proteins are lost in the urine. The loss of alpha 2 macroglobulin into urine is prevented by its large size. The net result is that alpha 2 macroglobulin reaches serum levels equal to or greater than those of albumin in the nephrotic syndrome, which has the effect of maintaining oncotic pressure.
REFERENCE
McPherson & Pincus: Henry's Clinical Diagnosis and Management by Laboratory Methods, 21st ed.
Firestein: Kelley's Textbook of Rheumatology, 8th edition.
Each monomer of Human alpha-2-macroglobulin is composed of many functional domains, including macroglobulin domains, a thiol ester-containing domain and a receptor-binding domain.
includes protease inhibitor
s, typified by the human tetrameric a2-macroglobulin (a2M); they belong to the MEROPS proteinase inhibitor family I39, clan IL. These protease inhibitors share several defining properties, which include (i) the ability to inhibit
protease
s from all catalytic classes, (ii) the presence of a 'bait region' and a thiol
ester, (iii) a similar protease inhibitory mechanism and (iv) the inactivation of the inhibitory capacity by reaction of the thiol ester
with small primary amine
s. aM protease inhibitor
s inhibit by steric hindrance. The mechanism involves protease cleavage
of the bait region, a segment of the aM that is particularly susceptible to proteolytic cleavage, which initiates a conformational change
such that the aM collapses about the protease. In the resulting aM-protease complex, the active site
of the protease is sterically
shielded, thus substantially decreasing access to protein
substrates
. Two additional events occur as a consequence of bait region cleavage, namely (i) the h-cysteinyl-g-glutamyl thiol ester becomes highly reactive and (ii) a major conformational
change exposes a conserved
COOH-terminal receptor
binding
domain (RBD). RBD exposure allows the aM protease complex
to bind to clearance receptors
and be removed from circulation. Tetrameric, dimeric, and, more recently, monomeric aM protease inhibitors have been identified.
Alpha-2-macroglobulin is able to inactivate an enormous variety of proteinases (including serine-, cysteine-, aspartic- and metalloproteinase
s). It functions as an inhibitor of fibrinolysis
by inhibiting plasmin
and kallikrein. It functions as an inhibitor of coagulation
by inhibiting thrombin
.
Alpha-2-macroglobulin has in its structure a 35 amino acid "bait" region. Proteinases binding and cleaving the bait region become bound to α2M. The proteinase-α2M complex is recognised by macrophage receptors and cleared from the system.
, a condition wherein the kidney
s start to leak out some of the smaller blood proteins. Because of its size, α2-macroglobulin is retained in the bloodstream. Increased production of all proteins means α2-macroglobulin concentration increases. This increase has little adverse effect on the health, but is used as a diagnostic clue. Longstanding chronic renal failure
can lead to amyloid
by alpha-2-macroglobulin (see main article: amyloid
).
A common variant (29.5%) (polymorphism
) of α2-macroglobulin leads to increased risk of Alzheimer's disease
,
α-2-macroglobulin binds to and removes the active forms of the gelatinase (MMP-2 and MMP-9) from the circulation via scavenger receptors on the phagocytes.
Blood
Blood is a specialized bodily fluid in animals that delivers necessary substances such as nutrients and oxygen to the cells and transports metabolic waste products away from those same cells....
. It is produced by the liver
Liver
The liver is a vital organ present in vertebrates and some other animals. It has a wide range of functions, including detoxification, protein synthesis, and production of biochemicals necessary for digestion...
, and is a major component of the alpha-2 band in protein electrophoresis
Protein electrophoresis
Protein electrophoresis is a method for analysing the proteins in a fluid or an extract. The electrophoresis may be performed with a small volume of sample in a number of alternative ways with or without a supporting medium: SDS polyacrylamide gel electrophoresis Protein electrophoresis is a method...
.
Alpha 2-Macroglobulin is the largest major nonimmunoglobulin protein in plasma. The alpha 2-macroglobulin molecule is synthesized mainly in liver, but also locally by macrophages, fibroblasts, and adrenocortical cells.
Alpha 2 macroglobulin acts as an antiprotease and is able to inactivate an enormous variety of proteinases. It functions as an inhibitor of fibrinolysis by inhibiting plasmin and kallikrein. It functions as an inhibitor of coagulation by inhibiting thrombin. Alpha 2-macroglobulin may act as a carrier protein because it also binds to numerous growth factors and cytokines, such as platelet-derived growth factor, basic fibroblast growth factor, TGF-β, insulin, and IL-1β.
No specific deficiency with associated disease has been recognized, and no disease state is attributed to low concentrations of Alpha 2 macroglobulin.
The concentration of alpha 2 macroglobulin rises 10-fold or more in the nephrotic syndrome when other lower molecular weight proteins are lost in the urine. The loss of alpha 2 macroglobulin into urine is prevented by its large size. The net result is that alpha 2 macroglobulin reaches serum levels equal to or greater than those of albumin in the nephrotic syndrome, which has the effect of maintaining oncotic pressure.
REFERENCE
McPherson & Pincus: Henry's Clinical Diagnosis and Management by Laboratory Methods, 21st ed.
Firestein: Kelley's Textbook of Rheumatology, 8th edition.
Structure
Human alpha-2-macroglobulin is composed of four identical subunits bound together by -S-S- bonds. In addition to tetrameric forms of alpha-2-macroglobulin, dimeric, and more recently monomeric aM protease inhibitors have been identified.Each monomer of Human alpha-2-macroglobulin is composed of many functional domains, including macroglobulin domains, a thiol ester-containing domain and a receptor-binding domain.
Function
The alpha-macroglobulin (aM) family of proteinsProtein family
A protein family is a group of evolutionarily-related proteins, and is often nearly synonymous with gene family. The term protein family should not be confused with family as it is used in taxonomy....
includes protease inhibitor
Protease inhibitor (biology)
In biology and biochemistry, protease inhibitors are molecules that inhibit the function of proteases. Many naturally occurring protease inhibitors are proteins....
s, typified by the human tetrameric a2-macroglobulin (a2M); they belong to the MEROPS proteinase inhibitor family I39, clan IL. These protease inhibitors share several defining properties, which include (i) the ability to inhibit
Enzyme inhibitor
An enzyme inhibitor is a molecule that binds to enzymes and decreases their activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used as herbicides and pesticides...
protease
Protease
A protease is any enzyme that conducts proteolysis, that is, begins protein catabolism by hydrolysis of the peptide bonds that link amino acids together in the polypeptide chain forming the protein....
s from all catalytic classes, (ii) the presence of a 'bait region' and a thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
ester, (iii) a similar protease inhibitory mechanism and (iv) the inactivation of the inhibitory capacity by reaction of the thiol ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
with small primary amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s. aM protease inhibitor
Protease inhibitor (biology)
In biology and biochemistry, protease inhibitors are molecules that inhibit the function of proteases. Many naturally occurring protease inhibitors are proteins....
s inhibit by steric hindrance. The mechanism involves protease cleavage
Cleavage
Cleavage may refer to:*Cleavage , partial exposure of the separation between a woman's breasts.**Cleavage enhancement, methods of making a person's breast cleavage look more substantial than it really is....
of the bait region, a segment of the aM that is particularly susceptible to proteolytic cleavage, which initiates a conformational change
Conformational change
A macromolecule is usually flexible and dynamic. It can change its shape in response to changes in its environment or other factors; each possible shape is called a conformation, and a transition between them is called a conformational change...
such that the aM collapses about the protease. In the resulting aM-protease complex, the active site
Active site
In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that...
of the protease is sterically
Steric effects
Steric effects arise from the fact that each atom within a molecule occupies a certain amount of space. If atoms are brought too close together, there is an associated cost in energy due to overlapping electron clouds , and this may affect the molecule's preferred shape and reactivity.-Steric...
shielded, thus substantially decreasing access to protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
. Two additional events occur as a consequence of bait region cleavage, namely (i) the h-cysteinyl-g-glutamyl thiol ester becomes highly reactive and (ii) a major conformational
Protein structure
Proteins are an important class of biological macromolecules present in all organisms. Proteins are polymers of amino acids. Classified by their physical size, proteins are nanoparticles . Each protein polymer – also known as a polypeptide – consists of a sequence formed from 20 possible L-α-amino...
change exposes a conserved
Conserved sequence
In biology, conserved sequences are similar or identical sequences that occur within nucleic acid sequences , protein sequences, protein structures or polymeric carbohydrates across species or within different molecules produced by the same organism...
COOH-terminal receptor
Receptor (biochemistry)
In biochemistry, a receptor is a molecule found on the surface of a cell, which receives specific chemical signals from neighbouring cells or the wider environment within an organism...
binding
Binding (molecular)
Molecular binding is an attractive interaction between two molecules which results in a stable association in which the molecules are in close proximity to each other...
domain (RBD). RBD exposure allows the aM protease complex
Protein complex
A multiprotein complex is a group of two or more associated polypeptide chains. If the different polypeptide chains contain different protein domain, the resulting multiprotein complex can have multiple catalytic functions...
to bind to clearance receptors
Receptor (biochemistry)
In biochemistry, a receptor is a molecule found on the surface of a cell, which receives specific chemical signals from neighbouring cells or the wider environment within an organism...
and be removed from circulation. Tetrameric, dimeric, and, more recently, monomeric aM protease inhibitors have been identified.
Alpha-2-macroglobulin is able to inactivate an enormous variety of proteinases (including serine-, cysteine-, aspartic- and metalloproteinase
Metalloproteinase
Metalloproteinases constitute a family of enzymes from the group of proteases, classified by the nature of the most prominent functional group in their active site. These are proteolytic enzymes whose catalytic mechanism involves a metal. Most metalloproteases are zinc-dependent, but some use...
s). It functions as an inhibitor of fibrinolysis
Fibrinolysis
Fibrinolysis is a process that prevents blood clots from growing and becoming problematic. This process has two types: primary fibrinolysis and secondary fibrinolysis...
by inhibiting plasmin
Plasmin
Plasmin is an important enzyme present in blood that degrades many blood plasma proteins, most notably, fibrin clots. The degradation of fibrin is termed fibrinolysis. In humans, the plasmin protein is encoded by the PLG gene.- Function :...
and kallikrein. It functions as an inhibitor of coagulation
Coagulation
Coagulation is a complex process by which blood forms clots. It is an important part of hemostasis, the cessation of blood loss from a damaged vessel, wherein a damaged blood vessel wall is covered by a platelet and fibrin-containing clot to stop bleeding and begin repair of the damaged vessel...
by inhibiting thrombin
Thrombin
Thrombin is a "trypsin-like" serine protease protein that in humans is encoded by the F2 gene. Prothrombin is proteolytically cleaved to form thrombin in the first step of the coagulation cascade, which ultimately results in the stemming of blood loss...
.
Alpha-2-macroglobulin has in its structure a 35 amino acid "bait" region. Proteinases binding and cleaving the bait region become bound to α2M. The proteinase-α2M complex is recognised by macrophage receptors and cleared from the system.

Disease
Alpha-2-macroglobulin levels are increased in nephrotic syndromeNephrotic syndrome
Nephrotic syndrome is a nonspecific disorder in which the kidneys are damaged, causing them to leak large amounts of protein from the blood into the urine....
, a condition wherein the kidney
Kidney
The kidneys, organs with several functions, serve essential regulatory roles in most animals, including vertebrates and some invertebrates. They are essential in the urinary system and also serve homeostatic functions such as the regulation of electrolytes, maintenance of acid–base balance, and...
s start to leak out some of the smaller blood proteins. Because of its size, α2-macroglobulin is retained in the bloodstream. Increased production of all proteins means α2-macroglobulin concentration increases. This increase has little adverse effect on the health, but is used as a diagnostic clue. Longstanding chronic renal failure
Chronic renal failure
Chronic kidney disease , also known as chronic renal disease, is a progressive loss in renal function over a period of months or years. The symptoms of worsening kidney function are unspecific, and might include feeling generally unwell and experiencing a reduced appetite...
can lead to amyloid
Amyloid
Amyloids are insoluble fibrous protein aggregates sharing specific structural traits. Abnormal accumulation of amyloid in organs may lead to amyloidosis, and may play a role in various neurodegenerative diseases.-Definition:...
by alpha-2-macroglobulin (see main article: amyloid
Amyloid
Amyloids are insoluble fibrous protein aggregates sharing specific structural traits. Abnormal accumulation of amyloid in organs may lead to amyloidosis, and may play a role in various neurodegenerative diseases.-Definition:...
).
A common variant (29.5%) (polymorphism
Polymorphism (biology)
Polymorphism in biology occurs when two or more clearly different phenotypes exist in the same population of a species — in other words, the occurrence of more than one form or morph...
) of α2-macroglobulin leads to increased risk of Alzheimer's disease
Alzheimer's disease
Alzheimer's disease also known in medical literature as Alzheimer disease is the most common form of dementia. There is no cure for the disease, which worsens as it progresses, and eventually leads to death...
,
α-2-macroglobulin binds to and removes the active forms of the gelatinase (MMP-2 and MMP-9) from the circulation via scavenger receptors on the phagocytes.
External links
- The MEROPSMeropsMerops may refer to:* Merops , a genus of bee-eaters.* MEROPS, an on-line database for peptidases.It may also refer to several figures from Greek mythology:* King of Ethiopia, husband of Clymene, who lay with Helios and bore Phaethon...
online database for peptidases and their inhibitors: I39.001

