
Accidental release source terms
Encyclopedia
Accidental release source terms are the mathematical equations that quantify the flow rate at which accidental releases of air pollutant
s into the ambient environment
can occur at industrial facilities such as petroleum refineries
, petrochemical
plants, natural gas
processing plants, oil and gas transportation pipelines
, chemical plants, and many other industrial activities. Governmental regulations in a good many countries require that the probability of such accidental releases be analyzed and their quantitative impact upon the environment and human health be determined so that mitigating steps can be planned and implemented.
There are a number of mathematical calculation methods for determining the flow rate at which gaseous and liquid pollutants might be released from various types of accidents. Such calculational methods are referred to as source terms, and this article on accidental release source terms explains some of the calculation methods used for determining the mass flow rate
at which gaseous pollutants may be accidentally released.
in a closed vessel is discharged to the atmosphere
through a hole or other opening, the gas velocity
through that opening may be choked (i.e., it has attained a maximum) or it may be non-choked.
Choked velocity, also referred to as sonic velocity, occurs when the ratio of the absolute source pressure to the absolute downstream pressure is equal to or greater than [(k + 1) ÷ 2 ] k÷(k - 1 ), where k is the specific heat ratio
of the discharged gas (sometimes called the isentropic expansion factor and sometimes denoted as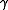 ).
).
For many gases, k ranges from about 1.09 to about 1.41, and therefore [(k + 1) ÷ 2 ] k÷(k - 1 ) ranges from 1.7 to about 1.9, which means that choked velocity usually occurs when the absolute source vessel pressure is at least 1.7 to 1.9 times as high as the absolute downstream ambient atmospheric pressure.
When the gas velocity is choked, the equation for the mass flow rate
in SI metric units is:
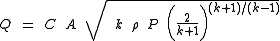
or this equivalent form:
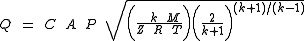
For the above equations, it is important to note that although the gas velocity reaches a maximum and becomes choked, the mass flow rate is not choked. The mass flow rate can still be increased if the source pressure is increased.
Whenever the ratio of the absolute source pressure to the absolute downstream ambient pressure is less than
[ ( k + 1 ) ÷ 2 ] k ÷ ( k - 1 ), then the gas velocity is non-choked (i.e., sub-sonic) and the equation for mass flow rate is:
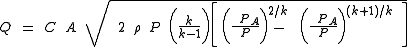
or this equivalent form:
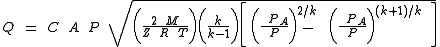
The above equations calculate the initial instantaneous mass flow rate for the pressure and temperature existing in the source vessel when a release first occurs. The initial instantaneous flow rate from a leak in a pressurized gas system or vessel is much higher than the average flow rate during the overall release period because the pressure and flow rate decrease with time as the system or vessel empties. Calculating the flow rate versus time since the initiation of the leak is much more complicated, but more accurate. Two equivalent methods for performing such calculations are presented and compared at www.air-dispersion.com/feature2.html.
The technical literature can be very confusing because many authors fail to explain whether they are using the universal gas law constant R which applies to any ideal gas
or whether they are using the gas law constant Rs which only applies to a specific individual gas. The relationship between the two constants is Rs = R/M.
Notes:
(1)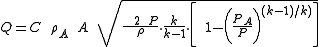
The gas density,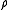 A, in Ramskill's equation is the ideal gas density at the downstream conditions of temperature and pressure and it is defined in equation (2) using the ideal gas law
A, in Ramskill's equation is the ideal gas density at the downstream conditions of temperature and pressure and it is defined in equation (2) using the ideal gas law
:
(2)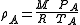
Since the downstream temperature TA is not known, the isentropic expansion equation below is used to determine TA in terms of the known upstream temperature T:
(3)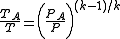
Combining equations (2) and (3) results in equation (4) which defines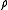 A in terms of the known upstream temperature T:
A in terms of the known upstream temperature T:
(4)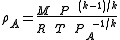
Using equation (4) with Ramskill's equation (1) to determine non-choked mass flow rates for ideal gases gives identical results to the results obtained using the non-choked flow equation presented in the previous section above.
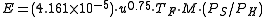
If TP = 0 °C or less, then TF = 1.0
If TP > 0 °C, then TF = 1.0 + 0.0043 TP2
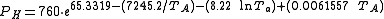
using units which were a mixture of metric usage and United States usage. The non-metric units have been converted to metric units for this presentation.
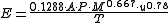
NB The constant used here is 0.284 from the mixed unit formula/2.205 lb/kg. The 82.05 become 1.0 = (ft/m)^2 x mmHg/kPa.
The U.S. EPA also defined the pool depth as 0.01 m (i.e., 1 cm) so that the surface area of the pool liquid could be calculated as:
Notes:
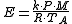
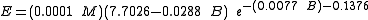
and the following equation, derived from a simple heat balance, is used to predict how much of the liquified gas is vaporized.
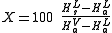
If the enthalpy data required for the above equation is unavailable, then the following equation may be used.
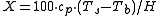
Pollutant
A pollutant is a waste material that pollutes air, water or soil, and is the cause of pollution.Three factors determine the severity of a pollutant: its chemical nature, its concentration and its persistence. Some pollutants are biodegradable and therefore will not persist in the environment in the...
s into the ambient environment
Natural environment
The natural environment encompasses all living and non-living things occurring naturally on Earth or some region thereof. It is an environment that encompasses the interaction of all living species....
can occur at industrial facilities such as petroleum refineries
Oil refinery
An oil refinery or petroleum refinery is an industrial process plant where crude oil is processed and refined into more useful petroleum products, such as gasoline, diesel fuel, asphalt base, heating oil, kerosene, and liquefied petroleum gas...
, petrochemical
Petrochemical
Petrochemicals are chemical products derived from petroleum. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as corn or sugar cane....
plants, natural gas
Natural gas
Natural gas is a naturally occurring gas mixture consisting primarily of methane, typically with 0–20% higher hydrocarbons . It is found associated with other hydrocarbon fuel, in coal beds, as methane clathrates, and is an important fuel source and a major feedstock for fertilizers.Most natural...
processing plants, oil and gas transportation pipelines
Pipeline transport
Pipeline transport is the transportation of goods through a pipe. Most commonly, liquids and gases are sent, but pneumatic tubes that transport solid capsules using compressed air are also used....
, chemical plants, and many other industrial activities. Governmental regulations in a good many countries require that the probability of such accidental releases be analyzed and their quantitative impact upon the environment and human health be determined so that mitigating steps can be planned and implemented.
There are a number of mathematical calculation methods for determining the flow rate at which gaseous and liquid pollutants might be released from various types of accidents. Such calculational methods are referred to as source terms, and this article on accidental release source terms explains some of the calculation methods used for determining the mass flow rate
Mass flow rate
Mass flow rate is the mass of substance which passes through a given surface per unit time. Its unit is mass divided by time, so kilogram per second in SI units, and slug per second or pound per second in US customary units...
at which gaseous pollutants may be accidentally released.
Accidental release of pressurized gas
When gas stored under pressurePressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
in a closed vessel is discharged to the atmosphere
Earth's atmosphere
The atmosphere of Earth is a layer of gases surrounding the planet Earth that is retained by Earth's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention , and reducing temperature extremes between day and night...
through a hole or other opening, the gas velocity
Velocity
In physics, velocity is speed in a given direction. Speed describes only how fast an object is moving, whereas velocity gives both the speed and direction of the object's motion. To have a constant velocity, an object must have a constant speed and motion in a constant direction. Constant ...
through that opening may be choked (i.e., it has attained a maximum) or it may be non-choked.
Choked velocity, also referred to as sonic velocity, occurs when the ratio of the absolute source pressure to the absolute downstream pressure is equal to or greater than [(k + 1) ÷ 2 ] k÷(k - 1 ), where k is the specific heat ratio
Heat capacity ratio
The heat capacity ratio or adiabatic index or ratio of specific heats, is the ratio of the heat capacity at constant pressure to heat capacity at constant volume . It is sometimes also known as the isentropic expansion factor and is denoted by \gamma or \kappa . The latter symbol kappa is...
of the discharged gas (sometimes called the isentropic expansion factor and sometimes denoted as
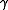 ).
).For many gases, k ranges from about 1.09 to about 1.41, and therefore [(k + 1) ÷ 2 ] k÷(k - 1 ) ranges from 1.7 to about 1.9, which means that choked velocity usually occurs when the absolute source vessel pressure is at least 1.7 to 1.9 times as high as the absolute downstream ambient atmospheric pressure.
When the gas velocity is choked, the equation for the mass flow rate
Mass flow rate
Mass flow rate is the mass of substance which passes through a given surface per unit time. Its unit is mass divided by time, so kilogram per second in SI units, and slug per second or pound per second in US customary units...
in SI metric units is:
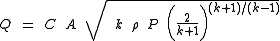
or this equivalent form:
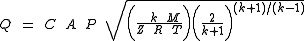
For the above equations, it is important to note that although the gas velocity reaches a maximum and becomes choked, the mass flow rate is not choked. The mass flow rate can still be increased if the source pressure is increased.
Whenever the ratio of the absolute source pressure to the absolute downstream ambient pressure is less than
[ ( k + 1 ) ÷ 2 ] k ÷ ( k - 1 ), then the gas velocity is non-choked (i.e., sub-sonic) and the equation for mass flow rate is:
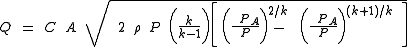
or this equivalent form:
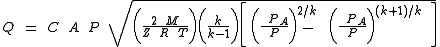
| where: | |
| Q | = mass flow rate Mass flow rate Mass flow rate is the mass of substance which passes through a given surface per unit time. Its unit is mass divided by time, so kilogram per second in SI units, and slug per second or pound per second in US customary units... , kg/s |
|---|---|
| C | = discharge coefficient, dimensionless (usually about 0.72) |
| A | = discharge hole area, m² |
| k | = cp/cv of the gas |
| cp | = specific heat of the gas at constant pressure |
| cv | = specific heat of the gas at constant volume |
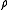 |
= real gas Ideal gas An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as... density Density The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight... at P and T, kg/m³ |
| P | = absolute upstream pressure, Pa |
| PA | = absolute ambient or downstream pressure, Pa |
| M | = the gas molecular mass Molecular mass The molecular mass of a substance is the mass of one molecule of that substance, in unified atomic mass unit u... , kg/kmol (also known as the molecular weight) |
| R | = the Universal Gas Law Constant Gas constant The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,... = 8314.5 Pa·m³/(kmol·K) |
| T | = absolute upstream gas temperature, K |
| Z | = the gas compressibility factor at P and T, dimensionless |
The above equations calculate the initial instantaneous mass flow rate for the pressure and temperature existing in the source vessel when a release first occurs. The initial instantaneous flow rate from a leak in a pressurized gas system or vessel is much higher than the average flow rate during the overall release period because the pressure and flow rate decrease with time as the system or vessel empties. Calculating the flow rate versus time since the initiation of the leak is much more complicated, but more accurate. Two equivalent methods for performing such calculations are presented and compared at www.air-dispersion.com/feature2.html.
The technical literature can be very confusing because many authors fail to explain whether they are using the universal gas law constant R which applies to any ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
or whether they are using the gas law constant Rs which only applies to a specific individual gas. The relationship between the two constants is Rs = R/M.
Notes:
- The above equations are for a real gas.
- For an ideal gas, Z = 1 and ρ is the ideal gas density.
- 1 kilomole (kmol) = 1000 molesMole (unit)The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
= 1000 gram-moles = kilogram-mole.
Ramskill's equation for non-choked mass flow
P.K. Ramskill's equation for the non-choked flow of an ideal gas is shown below as equation (1):(1)
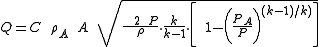
The gas density,
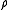 A, in Ramskill's equation is the ideal gas density at the downstream conditions of temperature and pressure and it is defined in equation (2) using the ideal gas law
A, in Ramskill's equation is the ideal gas density at the downstream conditions of temperature and pressure and it is defined in equation (2) using the ideal gas lawIdeal gas law
The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law...
:
(2)
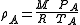
Since the downstream temperature TA is not known, the isentropic expansion equation below is used to determine TA in terms of the known upstream temperature T:
(3)
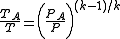
Combining equations (2) and (3) results in equation (4) which defines
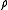 A in terms of the known upstream temperature T:
A in terms of the known upstream temperature T:(4)
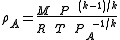
Using equation (4) with Ramskill's equation (1) to determine non-choked mass flow rates for ideal gases gives identical results to the results obtained using the non-choked flow equation presented in the previous section above.
Evaporation of non-boiling liquid pool
Three different methods of calculating the rate of evaporation from a non-boiling liquid pool are presented in this section. The results obtained by the three methods are somewhat different.The U.S. Air Force method
The following equations are for predicting the rate at which liquid evaporates from the surface of a pool of liquid which is at or near the ambient temperature. The equations were derived from field tests performed by the U.S. Air Force with pools of liquid hydrazine.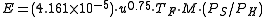
| where: | |
| E | = evaporation flux, (kg/min)/m² of pool surface |
|---|---|
| u | = windspeed just above the liquid surface, m/s |
| TA | = absolute ambient temperature, K |
| TF | = pool liquid temperature correction factor, dimensionless |
| TP | = pool liquid temperature, °C |
| M | = pool liquid molecular weight, dimensionless |
| PS | = pool liquid vapor pressure at ambient temperature, mmHg |
| PH | = hydrazine vapor pressure at ambient temperature, mmHg (see equation below) |
If TP = 0 °C or less, then TF = 1.0
If TP > 0 °C, then TF = 1.0 + 0.0043 TP2
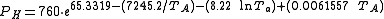
| where: | |
 |
= 2.7183, the base of the natural logarithm system |
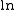 |
= natural logarithm |
The U.S. EPA method
The following equations are for predicting the rate at which liquid evaporates from the surface of a pool of liquid which is at or near the ambient temperature. The equations were developed by the United States Environmental Protection AgencyUnited States Environmental Protection Agency
The U.S. Environmental Protection Agency is an agency of the federal government of the United States charged with protecting human health and the environment, by writing and enforcing regulations based on laws passed by Congress...
using units which were a mixture of metric usage and United States usage. The non-metric units have been converted to metric units for this presentation.
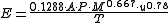
NB The constant used here is 0.284 from the mixed unit formula/2.205 lb/kg. The 82.05 become 1.0 = (ft/m)^2 x mmHg/kPa.
| where: | |
| E | = evaporation rate, kg/min |
|---|---|
| u | = windspeed just above the pool liquid surface, m/s |
| M | = pool liquid molecular weight, dimensionless |
| A | = surface area of the pool liquid, m² |
| P | = vapor pressure of the pool liquid at the pool temperature, kPa |
| T | = pool liquid absolute temperature, K |
The U.S. EPA also defined the pool depth as 0.01 m (i.e., 1 cm) so that the surface area of the pool liquid could be calculated as:
- A = (pool volume, in m³)/(0.01)
Notes:
- 1 kPa = 0.0102 kgfKGFKGF may refer to:*Keratinocyte Growth Factor*King George's Fields A UK set of 471 memorial playing fields and recreation grounds*Kolar Gold Fields*The IATA code for Sary-Arka Airport, Karaganda, Kazakhstan...
/cm² = 0.01 bar - mol = moleMole (unit)The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
- atm = atmosphere
Stiver and Mackay's method
The following equations are for predicting the rate at which liquid evaporates from the surface of a pool of liquid which is at or near the ambient temperature. The equations were developed by Warren Stiver and Dennis Mackay of the Chemical Engineering Department at the University of Toronto.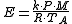
| where: | |
| E | = evaporation flux, (kg/s)/m² of pool surface |
|---|---|
| k | = mass transfer coefficient, m/s = 0.002 u |
| TA | = absolute ambient temperature, K |
| M | = pool liquid molecular weight, dimensionless |
| P | = pool liquid vapor pressure at ambient temperature, Pa |
| R | = the universal gas law constant = 8314.5 Pa·m³/(kmol·K) |
| u | = windspeed just above the liquid surface, m/s |
Evaporation of boiling cold liquid pool
The following equation is for predicting the rate at which liquid evaporates from the surface of a pool of cold liquid (i.e., at a liquid temperature of about 0 °C or less).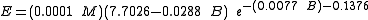
| where: | |
| E | = evaporation flux, (kg/min)/m² of pool surface |
|---|---|
| B | = pool liquid atmospheric boiling point, °C |
| M | = pool liquid molecular weight, dimensionless |
| e | = the base of the natural logarithm system = 2.7183 |
Adiabatic flash of liquified gas release
Liquified gases such as ammonia or chlorine are often stored in cylinders or vessels at ambient temperatures and pressures well above atmospheric pressure. When such a liquified gas is released into the ambient atmosphere, the resultant reduction of pressure causes some of the liquified gas to vaporize immediately. This is known as "adiabatic flashing"Flash evaporation
Flash evaporation is the partial vapor that occurs when a saturated liquid stream undergoes a reduction in pressure by passing through a throttling valve or other throttling device. This process is one of the simplest unit operations...
and the following equation, derived from a simple heat balance, is used to predict how much of the liquified gas is vaporized.
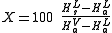
| where: | |
| X | = weight percent vaporized |
|---|---|
| HsL | = source liquid enthalpy Enthalpy Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a... at source temperature and pressure, J/kg |
| HaV | = flashed vapor enthalpy at atmospheric boiling point and pressure, J/kg |
| HaL | = residual liquid enthalpy at atmospheric boiling point and pressure, J/kg |
If the enthalpy data required for the above equation is unavailable, then the following equation may be used.
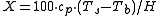
| where: | |
| X | = weight percent vaporized |
|---|---|
| cp | = source liquid specific heat, J/(kg °C) |
| Ts | = source liquid absolute temperature, K |
| Tb | = source liquid absolute atmospheric boiling point, K |
| H | = source liquid heat of vaporization at atmospheric boiling point, J/kg |
External links
- Ramskill's equations are presented and cited in this pdf file (use search function to find "Ramskill").
- More release source terms are available in the feature articles at www.air-dispersion.com
- Choked flow of gases
- Development of source emission models

