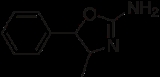
4-Methyl-aminorex
Encyclopedia
4-Methylaminorex is a stimulant
drug
of the 2-amino-5-aryl
oxazoline class that was first synthesized in the 1950s by McNeil Laboratories. It is also known by its street names "U4Euh" ("Euphoria") and "Ice". It is banned in many countries as a stimulant
.
4-Methylaminorex has effects comparable to methamphetamine
but with a much longer duration.
The results of animal experiments conducted with this drug suggest that it has an abuse liability similar to cocaine and amphetamine. One study found that, "stimulus properties of racemic cis, racemic trans, and all four individual optical isomers of 4-methylaminorex were examined in rats trained to discriminate 1 mg/kg of S(+)amphetamine sulfate from saline. The S(+)amphetamine stimulus generalized to all of the agents investigated". A second study in which rats trained to discriminate either 0.75 mg/kg S(+)-amphetamine or 1.5 mg/kg fenfluramine
from saline generalized to aminorex as amphetamine stimulus but not to fenfluramine. Rats trained to discriminate 8 mg/kg cocaine from saline generalized 4-methylaminorex to cocaine-stimulus. The reinforcing effects of cis-4-methylaminorex were determined in two models of intravenous drug self-administration in primates. Vehicle or 4-methylaminorex doses were substituted for cocaine. One of the two different doses of 4-methylaminorex maintained self-administration behavior above vehicle control levels.
The (±)-cis isomers [racemate (1:1-mixture) of the (4R,5S)-isomer and the enantiomeric (4S,5R)-isomer] generally synthesized from dl-phenylpropanolamine
in one step by cyclization with cyanogen bromide
(sometimes prepared in situ by reacting sodium cyanide
with bromine
). Alternate synthesis routes generally involve more steps, such as replacing cyanogen bromide with sodium or potassium cyanate
to form an intermediate and then reacting it with concentrated hydrochloric acid
. A method reported in microgram replaced the need for a separate addition of hydrochloric acid
by starting with the hydrochloride salt of the dl-phenylpropanolamine
but side-products are noted. The (±)-trans isomers [racemate (1:1-mixture) of the (4S,5S)-isomer and the enantiomeric (4R,5R)-isomer] are synthesized in the same manner above but dl-norpseudoephedrine is used as the starting material instead.
or taken orally.
As an anorectic
, the ED50
is 8.8 mg/kg in rats for the (±)-cis isomers. The (±)-trans isomers are slightly more potent at 7.0 mg/kg. As a recreational drug, the effective dosage ranges from 5 to 25 mg.
In the 1970s McNeil Laboratories
, Inc was trying to bring 4-methylaminorex to drug market as a sympathomimetic (most commonly used as asthma-medicines), research name was McN-822, they mention that human dose would have been 0.25 mg/kg of body weight. They mention also LD50: 17 mg/kg
p.o for mice
There is a patent about the use of 4-methylaminorex "as a nasal decongestant which, when administered orally, does not produce adverse central nervous system stimulant effects as experienced with other decongestants and anorexiants." Dose mentioned is 0.25 mg/kg of body weight.
. Large doses have been reported anecdotally to last up to 36 hours. The effects are stimulant
in nature, producing euphoria
, an increase in attention, and increased cognition
. Anecdotally, it has been reported to produce effects similar to nootropic
s, however, there is no research to support the claim that it is any different or more effective than other psychostimulants in this respect. Moreover, 4-methylaminorex does not have the established safety profile of widely-used clinical psychostimulants such as methylphenidate
, dextroamphetamine
and modafinil
.
There has been one reported death due to 4-methylaminorex and diazepam. Concentrations of 4-methylaminorex were: in blood 21.3 mg/L; in urine 12.3 mg/L. Diazepam concentration in blood was 0.8 mg/L. One rat study has studied excretion of 4-methylaminorex in urine:
"The concentration of trans-4-methylaminorex in rat urine following four injections of the trans-4S,5S isomer (5 mg/kg i.p each, at intervals of 12 h in 2 days, as measured quantitatively by GC/MS"
Also another study has studied pharmacokinetics and tissue distribution of the stereoisomers of 4-methylaminorex in rats.
"Pulmonary hypertension has been associated with ingestion of the appetite suppressant aminorex. A similar compound, 4-methylaminorex was discovered on the property of three individuals with diagnoses of pulmonary hypertension."
(TPH) activity (a possible marker for serotonin neurotoxicity) but citing study: "No change in TPH activity was observed 30 min after injection; by 8 h the activity of this enzyme appeared to be recovering." and "this agent is significantly less neurotoxic than methamphetamine
or MDMA."
Study published 2 years later than first one also suggested reduction in tryptophan hydroxylase activity, they used quite high dose too (10 mg/kg of cis-4-methylaminorex) and studied also long term effects (rats were killed 3 h, 18 h or 7 days after injection), they found reduction of 20-40% of tryptophan hydroxylase (TPH) activity and "recovery of TPH activity occurred 18 h after treatment, but was significantly decreased again by 7 days." but "It is noteworthy that, unlike the other analogs, the striatal levels of 5-HT did not decline with TPH activity following multiple 4-methylaminorex treatment"
Latest study (using mice) was not able to find any long term effects suggesting neurotoxicity and they found instead increase in serotonin levels, they used quite high doses too(15 mg/kg of each isomers studied) "The dosages used in the present experiments are about 6-10 times than the effective doses of aminorex
and stereoisomers inhibition of food intake." Doses were repeated 3 times a day and mice were killed 7 days after last dose. "Since a long-lasting depletion of dopamine or 5-HT appears to be a good predictor of dopamine or 5-HT neurotoxicity (Wagner et al. 1980; Ricaurte et al. 1985), the results suggest that the aminorex compounds except 4S,SS-dimethylaminorex, unlike MDMA or fenfluramine
, are not toxic to either dopamine or 5-HT neurotransmitter systems in CBA
mice. It was reported that although multiple doses of 4-methylaminorex caused long-term (7 days) declines in striatal tryptophan hydroxylase activity in SD rats, no changes were found in 5-HT and 5-HIAA levels (Hanson et al. 1992).
That first study [11] also suggested reduced dopamine (DA) levels (a possible marker for dopamine neurotoxicity), but citing study: "However, 8 h after drug administration no differences from control values were seen in DA
, DOPAC
or HVA
levels." and again later studies [12-13] didn't find any long term reduction.
, 4-Methylaminorex is a List I drug of the Opium Law
. It is not approved by the CBG, and so it is designated as lacking any medical use.
In Canada, 4-Methylaminorex is listed as Schedule III. In the United Kingdom, 4-Methylaminorex is listed as Class A. In Australia, 4-Methylaminorex is listed as Schedule 9, making it legal only for scientific and medical research.
In the United States, (±)-cis-4-methylaminorex was placed in Schedule I of the Controlled Substances Act
shortly after its emergence as a recreational drug in the mid 1980s. Manufacturing the trans isomer required a different process than those encountered when the substance was first scheduled, and was believed less potent than the cis isomer with a much lower abuse potential.
However, studies revealing the abuse potential of the 'trans' isomer, coupled with the development of new clandestine synthetic methods that would produce the trans created a potential loophole in the law, which covered only the 'cis' isomer.
To clarify the situation, the US Drug Enforcement Administration
published a paper in its DEA Microgram Journal, regarding interpretation of the relevant statutory law as it relates to the status of trans-4-methylaminorex. In summary, according to this non-legally binding decision, trans-4-methylaminorex is not currently a controlled substance, but a potential analog. In fact, the report explicitly states:
"The United States [Drug Enforcement Administration] has the following opinion on the legality of the positional isomer "trans"-4-methylaminorex, which, unlike its 'cis' isomer was never placed in any schedule under the Controlled Substances Act
."
However, the opinion does say that the agency considers the substance a potential controlled substance analog, making the substance identical to a Schedule I substance if intended for human consumption, according to the analog act. In fact, the report gives an account of a successful conviction under the analog act of an offense involving the trans isomer.
Stimulant
Stimulants are psychoactive drugs which induce temporary improvements in either mental or physical function or both. Examples of these kinds of effects may include enhanced alertness, wakefulness, and locomotion, among others...
drug
Drug
A drug, broadly speaking, is any substance that, when absorbed into the body of a living organism, alters normal bodily function. There is no single, precise definition, as there are different meanings in drug control law, government regulations, medicine, and colloquial usage.In pharmacology, a...
of the 2-amino-5-aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
oxazoline class that was first synthesized in the 1950s by McNeil Laboratories. It is also known by its street names "U4Euh" ("Euphoria") and "Ice". It is banned in many countries as a stimulant
Stimulant
Stimulants are psychoactive drugs which induce temporary improvements in either mental or physical function or both. Examples of these kinds of effects may include enhanced alertness, wakefulness, and locomotion, among others...
.
4-Methylaminorex has effects comparable to methamphetamine
Methamphetamine
Methamphetamine is a psychostimulant of the phenethylamine and amphetamine class of psychoactive drugs...
but with a much longer duration.
The results of animal experiments conducted with this drug suggest that it has an abuse liability similar to cocaine and amphetamine. One study found that, "stimulus properties of racemic cis, racemic trans, and all four individual optical isomers of 4-methylaminorex were examined in rats trained to discriminate 1 mg/kg of S(+)amphetamine sulfate from saline. The S(+)amphetamine stimulus generalized to all of the agents investigated". A second study in which rats trained to discriminate either 0.75 mg/kg S(+)-amphetamine or 1.5 mg/kg fenfluramine
Fenfluramine
Fenfluramine is a drug that was part of the Fen-Phen anti-obesity medication . Fenfluramine was introduced on the U.S. market in 1973. It is the racemic mixture of two enantiomers, dextrofenfluramine and levofenfluramine...
from saline generalized to aminorex as amphetamine stimulus but not to fenfluramine. Rats trained to discriminate 8 mg/kg cocaine from saline generalized 4-methylaminorex to cocaine-stimulus. The reinforcing effects of cis-4-methylaminorex were determined in two models of intravenous drug self-administration in primates. Vehicle or 4-methylaminorex doses were substituted for cocaine. One of the two different doses of 4-methylaminorex maintained self-administration behavior above vehicle control levels.
Chemistry
4-Methylaminorex exists as four stereoisomers - (±)-cis and (±)-trans. The (±)-cis isomers are the form used recreationally.The (±)-cis isomers [racemate (1:1-mixture) of the (4R,5S)-isomer and the enantiomeric (4S,5R)-isomer] generally synthesized from dl-phenylpropanolamine
Phenylpropanolamine
Phenylpropanolamine , also known as the stereoisomers norephedrine and norpseudoephedrine, is a psychoactive drug of the phenethylamine and amphetamine chemical classes which is used as a stimulant, decongestant, and anorectic agent. It is commonly used in prescription and over-the-counter cough...
in one step by cyclization with cyanogen bromide
Cyanogen bromide
Cyanogen bromide is a pseudohalogen compound with the formula CNBr. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides, and synthesize other compounds.-Synthesis, basic properties, and structure:...
(sometimes prepared in situ by reacting sodium cyanide
Sodium cyanide
Sodium cyanide is an inorganic compound with the formula NaCN. This highly toxic colorless salt is used mainly in gold mining but has other niche applications...
with bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
). Alternate synthesis routes generally involve more steps, such as replacing cyanogen bromide with sodium or potassium cyanate
Cyanate
The cyanate ion is an anion with the chemical formula written as [OCN]− or [NCO]−. In aqueous solution it acts as a base, forming isocyanic acid, HNCO. The cyanate ion is an ambidentate ligand, forming complexes with a metal ion in which either the nitrogen or oxygen atom may be the electron-pair...
to form an intermediate and then reacting it with concentrated hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
. A method reported in microgram replaced the need for a separate addition of hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
by starting with the hydrochloride salt of the dl-phenylpropanolamine
Phenylpropanolamine
Phenylpropanolamine , also known as the stereoisomers norephedrine and norpseudoephedrine, is a psychoactive drug of the phenethylamine and amphetamine chemical classes which is used as a stimulant, decongestant, and anorectic agent. It is commonly used in prescription and over-the-counter cough...
but side-products are noted. The (±)-trans isomers [racemate (1:1-mixture) of the (4S,5S)-isomer and the enantiomeric (4R,5R)-isomer] are synthesized in the same manner above but dl-norpseudoephedrine is used as the starting material instead.
Dosage
4-methylaminorex can be smoked, insufflatedInsufflation (medicine)
Insufflation is the practice of inhaling a substance. Insufflation has limited medical use, but is a common route of administration with many respiratory drugs used to treat conditions in the lungs and paranasal sinus .The technique is common for many recreational drugs and is also used for some...
or taken orally.
As an anorectic
Anorectic
An anorectic or anorexic , also known as anorexigenic or appetite suppressant, is a dietary supplement and/or drug which reduces appetite, food consumption, and as a result, causes weight loss to occur.-List of anorectics:Numerous pharmaceutical compounds are marketed as appetite suppressants.The...
, the ED50
Effective dose (pharmacology)
An effective dose in pharmacology is the dose or amount of drug that produces a therapeutic response or desired effect in some fraction of the subjects taking it....
is 8.8 mg/kg in rats for the (±)-cis isomers. The (±)-trans isomers are slightly more potent at 7.0 mg/kg. As a recreational drug, the effective dosage ranges from 5 to 25 mg.
In the 1970s McNeil Laboratories
McNeil Laboratories
McNeil Consumer Healthcare is a medicals products company belonging to the Johnson & Johnson healthcare products group.-History:The company was founded on March 16, 1879 by 23-year-old Robert McNeil, who paid $167 for a drugstore complete with fixtures, inventory and soda fountain, as a retail...
, Inc was trying to bring 4-methylaminorex to drug market as a sympathomimetic (most commonly used as asthma-medicines), research name was McN-822, they mention that human dose would have been 0.25 mg/kg of body weight. They mention also LD50: 17 mg/kg
p.o for mice
There is a patent about the use of 4-methylaminorex "as a nasal decongestant which, when administered orally, does not produce adverse central nervous system stimulant effects as experienced with other decongestants and anorexiants." Dose mentioned is 0.25 mg/kg of body weight.
Effects
It produces long-lasting effects, generally up to 16 hours in duration if taken orally and up to 12 hours if smoked or insufflatedInsufflation (medicine)
Insufflation is the practice of inhaling a substance. Insufflation has limited medical use, but is a common route of administration with many respiratory drugs used to treat conditions in the lungs and paranasal sinus .The technique is common for many recreational drugs and is also used for some...
. Large doses have been reported anecdotally to last up to 36 hours. The effects are stimulant
Stimulant
Stimulants are psychoactive drugs which induce temporary improvements in either mental or physical function or both. Examples of these kinds of effects may include enhanced alertness, wakefulness, and locomotion, among others...
in nature, producing euphoria
Euphoria (emotion)
Euphoria is medically recognized as a mental and emotional condition in which a person experiences intense feelings of well-being, elation, happiness, ecstasy, excitement and joy...
, an increase in attention, and increased cognition
Cognition
In science, cognition refers to mental processes. These processes include attention, remembering, producing and understanding language, solving problems, and making decisions. Cognition is studied in various disciplines such as psychology, philosophy, linguistics, and computer science...
. Anecdotally, it has been reported to produce effects similar to nootropic
Nootropic
Nootropics , also referred to as smart drugs, brain steroids, memory enhancers, cognitive enhancers, and intelligence enhancers, are drugs, supplements, nutraceuticals, and functional foods that improve mental functions such as cognition, memory, intelligence, motivation, attention, and concentration...
s, however, there is no research to support the claim that it is any different or more effective than other psychostimulants in this respect. Moreover, 4-methylaminorex does not have the established safety profile of widely-used clinical psychostimulants such as methylphenidate
Methylphenidate
Methylphenidate is a psychostimulant drug approved for treatment of attention-deficit hyperactivity disorder, postural orthostatic tachycardia syndrome and narcolepsy. It may also be prescribed for off-label use in treatment-resistant cases of lethargy, depression, neural insult and obesity...
, dextroamphetamine
Dextroamphetamine
Dextroamphetamine is a psychostimulant drug which is known to produce increased wakefulness and focus as well as decreased fatigue and decreased appetite....
and modafinil
Modafinil
Modafinil is an analeptic drug manufactured by Cephalon, and is approved by the U.S. Food and Drug Administration for the treatment of narcolepsy, shift work sleep disorder, and excessive daytime sleepiness associated with obstructive sleep apnea...
.
| Time (h) | Concentration of 4-methylaminorex in urine (µg/ml) |
|---|---|
| 0-6 | 45 |
| 6-24 | 1.0 |
| 24-36 | 0.1 |
| 36-48 | not detected |
There has been one reported death due to 4-methylaminorex and diazepam. Concentrations of 4-methylaminorex were: in blood 21.3 mg/L; in urine 12.3 mg/L. Diazepam concentration in blood was 0.8 mg/L. One rat study has studied excretion of 4-methylaminorex in urine:
"The concentration of trans-4-methylaminorex in rat urine following four injections of the trans-4S,5S isomer (5 mg/kg i.p each, at intervals of 12 h in 2 days, as measured quantitatively by GC/MS"
Also another study has studied pharmacokinetics and tissue distribution of the stereoisomers of 4-methylaminorex in rats.
"Pulmonary hypertension has been associated with ingestion of the appetite suppressant aminorex. A similar compound, 4-methylaminorex was discovered on the property of three individuals with diagnoses of pulmonary hypertension."
Possible neurotoxicity
There have been three studies studying possible neurotoxicity of 4-methylaminorex. First study using quite high doses (highest dose caused clonic seizures and some rats died) in rats and studying short term effects (rats were killed 30 min to 18 h after injection of 5, 10 or 20 mg/kg of racemic cis-4-methylaminorex) suggested reduction in tryptophan hydroxylaseTryptophan hydroxylase
Tryptophan hydroxylase is an enzyme involved in the synthesis of the neurotransmitter serotonin. TPH catalyzes the following chemical reactionIt employs one cofactor, iron.- Function :...
(TPH) activity (a possible marker for serotonin neurotoxicity) but citing study: "No change in TPH activity was observed 30 min after injection; by 8 h the activity of this enzyme appeared to be recovering." and "this agent is significantly less neurotoxic than methamphetamine
Methamphetamine
Methamphetamine is a psychostimulant of the phenethylamine and amphetamine class of psychoactive drugs...
or MDMA."
Study published 2 years later than first one also suggested reduction in tryptophan hydroxylase activity, they used quite high dose too (10 mg/kg of cis-4-methylaminorex) and studied also long term effects (rats were killed 3 h, 18 h or 7 days after injection), they found reduction of 20-40% of tryptophan hydroxylase (TPH) activity and "recovery of TPH activity occurred 18 h after treatment, but was significantly decreased again by 7 days." but "It is noteworthy that, unlike the other analogs, the striatal levels of 5-HT did not decline with TPH activity following multiple 4-methylaminorex treatment"
Latest study (using mice) was not able to find any long term effects suggesting neurotoxicity and they found instead increase in serotonin levels, they used quite high doses too(15 mg/kg of each isomers studied) "The dosages used in the present experiments are about 6-10 times than the effective doses of aminorex
Aminorex
Aminorex is an anorectic stimulant drug of the 2-amino-5-aryl oxazoline class developed by a team at McNeil in 1962. It is closely related to 4-methylaminorex. Aminorex has been shown to have locomotor stimulant effects, lying midway between dextroamphetamine and methamphetamine...
and stereoisomers inhibition of food intake." Doses were repeated 3 times a day and mice were killed 7 days after last dose. "Since a long-lasting depletion of dopamine or 5-HT appears to be a good predictor of dopamine or 5-HT neurotoxicity (Wagner et al. 1980; Ricaurte et al. 1985), the results suggest that the aminorex compounds except 4S,SS-dimethylaminorex, unlike MDMA or fenfluramine
Fenfluramine
Fenfluramine is a drug that was part of the Fen-Phen anti-obesity medication . Fenfluramine was introduced on the U.S. market in 1973. It is the racemic mixture of two enantiomers, dextrofenfluramine and levofenfluramine...
, are not toxic to either dopamine or 5-HT neurotransmitter systems in CBA
CBA
- Terminology :* Collective bargaining agreement** NBA Collective Bargaining Agreement** NHL Collective Bargaining Agreement* Consensus based assessment* Cost-benefit analysis* Cytometric Bead Array, a bead-based immunoassay- Institutions :...
mice. It was reported that although multiple doses of 4-methylaminorex caused long-term (7 days) declines in striatal tryptophan hydroxylase activity in SD rats, no changes were found in 5-HT and 5-HIAA levels (Hanson et al. 1992).
That first study [11] also suggested reduced dopamine (DA) levels (a possible marker for dopamine neurotoxicity), but citing study: "However, 8 h after drug administration no differences from control values were seen in DA
Dopamine
Dopamine is a catecholamine neurotransmitter present in a wide variety of animals, including both vertebrates and invertebrates. In the brain, this substituted phenethylamine functions as a neurotransmitter, activating the five known types of dopamine receptors—D1, D2, D3, D4, and D5—and their...
, DOPAC
Dopa
Dopa or DOPA can refer to:* L-DOPA , used in the treatment of Parkinson's disease* D-DOPA, a chemical compound related to L-DOPA* Dopa, an angel in Enochian* Deleting Online Predators Act of 2006...
or HVA
Homovanillic acid
Homovanillic acid is a major catecholamine metabolite...
levels." and again later studies [12-13] didn't find any long term reduction.
Legal Status
In the NetherlandsNetherlands
The Netherlands is a constituent country of the Kingdom of the Netherlands, located mainly in North-West Europe and with several islands in the Caribbean. Mainland Netherlands borders the North Sea to the north and west, Belgium to the south, and Germany to the east, and shares maritime borders...
, 4-Methylaminorex is a List I drug of the Opium Law
Opium Law
The Opium Law is the section of the Dutch law which covers nearly all psychotropic drugs. All non-psychotropic, but prescription-only drugs are covered by the Medicine Act.- Origin and history :...
. It is not approved by the CBG, and so it is designated as lacking any medical use.
In Canada, 4-Methylaminorex is listed as Schedule III. In the United Kingdom, 4-Methylaminorex is listed as Class A. In Australia, 4-Methylaminorex is listed as Schedule 9, making it legal only for scientific and medical research.
In the United States, (±)-cis-4-methylaminorex was placed in Schedule I of the Controlled Substances Act
Controlled Substances Act
The Controlled Substances Act was enacted into law by the Congress of the United States as Title II of the Comprehensive Drug Abuse Prevention and Control Act of 1970. The CSA is the federal U.S. drug policy under which the manufacture, importation, possession, use and distribution of certain...
shortly after its emergence as a recreational drug in the mid 1980s. Manufacturing the trans isomer required a different process than those encountered when the substance was first scheduled, and was believed less potent than the cis isomer with a much lower abuse potential.
However, studies revealing the abuse potential of the 'trans' isomer, coupled with the development of new clandestine synthetic methods that would produce the trans created a potential loophole in the law, which covered only the 'cis' isomer.
To clarify the situation, the US Drug Enforcement Administration
Drug Enforcement Administration
The Drug Enforcement Administration is a federal law enforcement agency under the United States Department of Justice, tasked with combating drug smuggling and use within the United States...
published a paper in its DEA Microgram Journal, regarding interpretation of the relevant statutory law as it relates to the status of trans-4-methylaminorex. In summary, according to this non-legally binding decision, trans-4-methylaminorex is not currently a controlled substance, but a potential analog. In fact, the report explicitly states:
"The United States [Drug Enforcement Administration] has the following opinion on the legality of the positional isomer "trans"-4-methylaminorex, which, unlike its 'cis' isomer was never placed in any schedule under the Controlled Substances Act
Controlled Substances Act
The Controlled Substances Act was enacted into law by the Congress of the United States as Title II of the Comprehensive Drug Abuse Prevention and Control Act of 1970. The CSA is the federal U.S. drug policy under which the manufacture, importation, possession, use and distribution of certain...
."
However, the opinion does say that the agency considers the substance a potential controlled substance analog, making the substance identical to a Schedule I substance if intended for human consumption, according to the analog act. In fact, the report gives an account of a successful conviction under the analog act of an offense involving the trans isomer.
See also
- AminorexAminorexAminorex is an anorectic stimulant drug of the 2-amino-5-aryl oxazoline class developed by a team at McNeil in 1962. It is closely related to 4-methylaminorex. Aminorex has been shown to have locomotor stimulant effects, lying midway between dextroamphetamine and methamphetamine...
- ClominorexClominorexClominorex is a centrally acting sympathomimetic which is related to other drugs such as aminorex and pemoline. It was developed as an appetite suppressant by McNeil Laboratories in the 1950s.-See also:* 4-Methylaminorex* Aminorex* Cyclazodone...
- CyclazodoneCyclazodoneCyclazodone is a centrally acting stimulant drug developed by American Cyanamid Company in the 1960s. The drug is related to other drugs such as pemoline and 4-methylaminorex. It is a banned stimulant under the World Anti-Doping Agency prohibited list....
- FenozoloneFenozoloneFenozolone was developed by Laboratoires Dausse in the 1960s and is a psychoactive drug and stimulant related to pemoline and 4-methylaminorex which acts as a norepinephrine-dopamine releasing agent .-See also:* 4-Methylaminorex...
- FluminorexFluminorexFluminorex is a centrally acting sympathomimetic which is related to other drugs such as aminorex and pemoline. It was developed as an appetite suppressant by McNeil Laboratories in the 1950s.-See also:* 4-Methylaminorex* Aminorex* Clominorex...
- PemolinePemolinePemoline was first synthesized in 1913 but it's activity was not discovered until the 1930s. Under the names it was used as a medication used to treat attention-deficit hyperactivity disorder and narcolepsy. Under the Convention on Psychotropic Substances, it is a Schedule IV drug...
- ThozalinoneThozalinoneThozalinone is a psychostimulant that has been used as an antidepressant in Europe. It has also been trialed as an anorectic. Thozalinone likely acts via inducing the release of norepinephrine and dopamine as with its analogues pemoline and aminorex.- References :...

