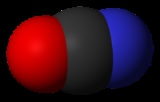
Cyanate
Encyclopedia

Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
written as [OCN]− or [NCO]−. In aqueous solution it acts as a base, forming isocyanic acid
Isocyanic acid
Isocyanic acid is an inorganic compound with the formula HNCO, discovered in 1830 by Liebig and Wöhler. This colourless substance is volatile and poisonous, with a boiling point of 23.5 °C...
, HNCO. The cyanate ion is an ambidentate ligand, forming complexes with a metal ion in which either the nitrogen or oxygen atom may be the electron-pair donor. It can also act as a bridging ligand
Bridging ligand
A bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are...
. Organic cyanates are called isocyanate
Isocyanate
Isocyanate is the functional group of elements –N=C=O , not to be confused with the cyanate functional group which is arranged as –O–C≡N or with isocyanide, R-N≡C. Any organic compound which contains an isocyanate group may also be referred to in brief as an isocyanate. An isocyanate may have more...
s when there is a C-NCO bond and cyanate ester
Cyanate ester
Cyanate esters are chemical substances generally based on a bisphenol or novolac derivative, in which the hydrogen atom of the phenolic OH group is substituted by a cyanide group...
s when there is a C-OCN bond.
Cyanate ion
The three atoms in a cyanate ion lie on a straight line, giving the ion a linear structure. The electronic structure is described most simply as- :Ö:-C≡N:
with a single C-O bond and a triple C-N bond. The infrared spectrum of a cyanate salt has a band at ca. 2096 cm−1; such a high frequency is characteristic of a triple bond
Triple bond
A triple bond in chemistry is a chemical bond between two chemical elements involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are...
. The cyanate ion is a Lewis base. Both the oxygen and nitrogen atoms carry a lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
of electrons and either one or the other, or both can be donated to Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
acceptors. It can be described as an ambidentate ligand.
Acid-base properties
Isocyanic acidIsocyanic acid
Isocyanic acid is an inorganic compound with the formula HNCO, discovered in 1830 by Liebig and Wöhler. This colourless substance is volatile and poisonous, with a boiling point of 23.5 °C...
, HNCO, is produced when a cyanate salt is acidified. Although the electronic structure according to valence bond theory
Valence bond theory
In chemistry, valence bond theory is one of two basic theories, along with molecular orbital theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds...
can be written as HN=C=O, as illustrated, the vibrational spectrum has a band at 2259 cm−1 which clearly requires the C-N bond to be a triple bond. In valence bond theory the canonical form HN+≡C-O- is the major contributor to the resonance hybrid.
The pure compound has a melting point
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
of -86.8°C and a boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
of 23.5°C, so it is volatile at ambient temperatures. In aqueous solution it is a weak acid
Weak acid
A weak acid is an acid that dissociates incompletely. It does not release all of its hydrogens in a solution, donating only a partial amount of its protons to the solution...
.
- HNCO H+ + NCO-; pKaAcid dissociation constantAn acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
= ca. 3.7.
On heating isocyanic acid is converted to the trimer
Trimer (chemistry)
In chemistry, a trimer is a product derived from three identical precursors. Trimers are typically cyclic. Chemical compounds that often trimerise are aliphatic isocyanates and cyanic acids. Often, trimerization competes with polymerization....
cyanuric acid
Cyanuric acid
Cyanuric acid or 1,3,5-triazine-2,4,6-triol is a chemical compound with the formula 3. Like many industrially useful chemicals, this triazine has many synonyms. This white, odorless solid finds use as a precursor or a component of bleaches, disinfectants, and herbicides...
, which itself decomposes on further heating back to isocyanic acid. In the reverse of the famous synthesis of urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
by Friedrich Wöhler
Friedrich Wöhler
Friedrich Wöhler was a German chemist, best known for his synthesis of urea, but also the first to isolate several chemical elements.-Biography:He was born in Eschersheim, which belonged to aau...
,
- OC(NH2)2 (heat)→ HNCO + NH3
isocyanic acid is produced and rapidly trimerizes to cyanuric acid.
The tautomer
Tautomer
Tautomers are isomers of organic compounds that readily interconvert by a chemical reaction called tautomerization. This reaction commonly results in the formal migration of a hydrogen atom or proton, accompanied by a switch of a single bond and adjacent double bond...
, known as cyanic acid, NCOH , in which the oxygen atom is protonated, is unstable to decomposition, but in solution it is present in equilibrium with isocyanic acid to the extent of about 3%. The vibrational spectrum is indicative of the presence of a triple bond between the nitrogen and carbon atoms.
Cyanate salts
Sodium cyanate is isostructural with sodium azideSodium azide
Sodium azide is the inorganic compound with the formula NaN3. This colourless azide salt is the gas-forming component in many car airbag systems. It is used for the preparation of other azide compounds. It is an ionic substance and is highly soluble in water. It is extremely...
, confirming the linear structure of the cyanate ion. It is made industrially by heating a mixture of sodium carbonate
Sodium carbonate
Sodium carbonate , Na2CO3 is a sodium salt of carbonic acid. It most commonly occurs as a crystalline heptahydrate, which readily effloresces to form a white powder, the monohydrate. Sodium carbonate is domestically well-known for its everyday use as a water softener. It can be extracted from the...
and urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
.
- Na2CO3 + OC(NH2)2 → 2NaNCO + CO2 + 2NH3 + H2O
A similar reaction is used to make potassium cyanate
Potassium cyanate
Potassium cyanate is an inorganic compound with the formula KOCN . It is a colourless solid. It is used to prepare many other compounds including useful herbicide. Worldwide production of the potassium and sodium salts was 20,000 tons in 2006.-Structure and bonding:Cyanate is isoelectronic with...
. Cyanates are produced when cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
s are oxidized. Use of this fact is dnien made in cyanide decontamination processes where oxidants such as permanganate
Permanganate
A permanganate is the general name for a chemical compound containing the manganate ion, . Because manganese is in the +7 oxidation state, the permanganate ion is a strong oxidizing agent. The ion has tetrahedral geometry...
and hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
are used to convert toxic cyanide to safer cyanate.
Complexes with the cyanate ion
Cyanate is an ambidentate ligand which can donate the pair of electrons on the nitrogen atom or the oxygen atom, or both. Structurally the isomers can be distinguished by the geometry of the complex. In N-bonded cyanate complexes the M-NCO unit has a linear structure, but with O-bonded cyanate the M-O-C unit is bent. Thus, the silver cyanato complex, [Ag(NCO)2]-, has a linear structure, so is N-bonded.Infrared spectroscopy
Infrared spectroscopy
Infrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
has been used extensively to distinguish between isomers. Many complexes of divalent
Divalent
In chemistry, a divalent ion or molecule has a valence of two and thus can form two bonds with other ions or molecules. An older term for divalent is bivalent....
metals are N-bonded. O-bonding has been suggested for complexes of the type [M(OCN)6]n-, M=Mo(III), Re(IV) and Re(V). The yellow complex Rh(PPh3)3(NCO) and orange complex Rh(PPh3)3(OCN) are linkage isomers and show differences in their infrared spectra which can be used for diagnosis (PPh3 stands for triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
).
The cyanate ion can bridge between two metal atoms by using both donor atoms. For example, this structure is found in the compound [Ni2(NCO)2(en)2][BPh4]2 (en = ethylene diamine
Ethylene diamine
Ethylenediamine is the organic compound with the formula C2H42. This colorless liquid with an ammonia-like odor is a strongly basic amine. The liquid fumes upon contact with humid air...
, BPh4 = tetraphenylborate
Tetraphenylborate
Tetraphenylborate is an organoboron anion consisting of a central boron atom with four phenyl groups. Tetraphenylborate uncouples oxidative phosphorylation....
). In this compound both the Ni-N-C unit and Ni-O-C unit are bent, even though in the first case donation is through the nitrogen atom.
Cyanate in organic compounds
Organic compoundOrganic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s that contain the functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
~N=C=O are known as isocyanate
Isocyanate
Isocyanate is the functional group of elements –N=C=O , not to be confused with the cyanate functional group which is arranged as –O–C≡N or with isocyanide, R-N≡C. Any organic compound which contains an isocyanate group may also be referred to in brief as an isocyanate. An isocyanate may have more...
s. It is conventional in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
to write isocyanates with two double bonds, which accords with a simplistic valence bond theory
Valence bond theory
In chemistry, valence bond theory is one of two basic theories, along with molecular orbital theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds...
of the bonding. In nucleophilic substitution
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
reactions cyanate usually forms an isocyanate. Isocyanates are widely used in the manufacture of polyurethane
Polyurethane
A polyurethane is any polymer composed of a chain of organic units joined by carbamate links. Polyurethane polymers are formed through step-growth polymerization, by reacting a monomer with another monomer in the presence of a catalyst.Polyurethanes are...
products and pesticide
Pesticide
Pesticides are substances or mixture of substances intended for preventing, destroying, repelling or mitigating any pest.A pesticide may be a chemical unicycle, biological agent , antimicrobial, disinfectant or device used against any pest...
s; Methyl isocyanate
Methyl isocyanate
Methyl isocyanate is an organic compound with the molecular formula CH3NCO. Synonyms are isocyanatomethane, methyl carbylamine, and MIC. Methyl isocyanate is an intermediate chemical in the production of carbamate pesticides . It has also been used in the production of rubbers and adhesives...
, used to make pesticides, was a major factor in the Bhopal disaster
Bhopal disaster
The Bhopal disaster also known as Bhopal Gas Tragedy was a gas leak incident in India, considered one of the world's worst industrial catastrophes. It occurred on the night of December 2–3, 1984 at the Union Carbide India Limited pesticide plant in Bhopal, Madhya Pradesh, India...
.
Compounds that contain the group ~O-C≡N, are known as a cyanates, or cyanate ester
Cyanate ester
Cyanate esters are chemical substances generally based on a bisphenol or novolac derivative, in which the hydrogen atom of the phenolic OH group is substituted by a cyanide group...
s. Aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
cyanates such are phenyl cyanate, C6H5OCN, can be formed by a reaction of phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
with cyanogen chloride
Cyanogen chloride
Cyanogen chloride is an inorganic compound with the formula NCCl. This linear, triatomic pseudohalogen is an easily condensed colorless gas. More commonly encountered in the laboratory is the related compound cyanogen bromide, a room-temperature solid that is widely used in biochemical analysis and...
, ClCN, in the presence of a base.

