Transition metal carbene complex
Encyclopedia
A transition metal carbene complex is a organometallic compound featuring a divalent organic ligand. The divalent organic ligand coordinated to the metal center is called a carbene
. Carbene complexes for almost all transition metal
s have been reported. Many methods for synthesizing them and reactions utilizing them have been reported. The term carbene ligand is a formalism since many are not derived from carbenes and almost none exhibit the reactivity characteristic of carbenes. Described often as M=CR2, they represent a class of organic ligands intermediate between alkyls (-CR3) and carbynes (≡CR). They feature in many catalytic reactions in the petrochemical industry and are of increasing interest in fine chemicals.
The characterization of (CO)5Cr(COCH3(Ph)) in the 1960s is often cited as the starting point of the area,
although carbenoid ligands had been previously implicated.
feature strong π-acceptors at the metal and being electrophilic
at the carbene carbon atom. Schrock carbenes, named after Richard R. Schrock
, are characterized by more nucleophilic carbene carbon centers, these species typically feature higher valent metals. N-heterocyclic carbenes (NHCs) were popularlized following Arduengo's isolation of a stable free carbene in 1991. Reflecting the growth of the area, carbene complexes are now known with a broad range of different reactivities and diverse substituents. Often it is not possible to classify a carbene complex with regards to its electrophilicity or nucleophilicity.
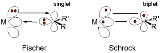
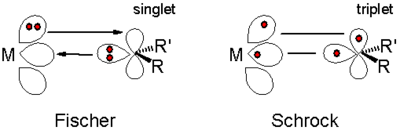
The chemical bond
ing (scheme 1) is based on electron δ-type
donation group of the filled methylene lone pair
orbital to an empty metal d-orbital, and pi electron back bonding of a filled metal d-orbital to the empty p-orbital on carbon. An example is the complex (CO)5Cr=C(NR2)Ph.
Fischer carbenes can be likened to ketones, with the carbene carbon being electrophilic, much like the carbonyl carbon of a ketone. Like ketones, Fischer carbene species can undergo Aldol
-like reactions. The hydrogen atoms attached to the carbon α to the carbene carbon are acidic, and can be deprotonated by a base such as n-butyllithium
, to give a nucleophile which can undergo further reaction.
This carbene is also the starting material for other reactions such as the Wulff-Dötz reaction. These type of carbenes were discovered by E. O. Fischer, and together with other achievements in organometalic chemistry, he was awarded the Nobel prize.
. Schrock carbenes are typically found with:
Bonding in such complexes can be viewed as the coupling of a triplet state metal and triplet carbene. These bonds are polarized towards carbon and therefore the methylene group is a nucleophile. An example of a Schrock carbene is the compound Ta(=C(H)But)(CH2But)3, with a tantalum(V) center doubly bonded to a neopentylidene ligand as well as three neopentyl ligands. An example of interest in organic synthesis is Tebbe's reagent
.
s, which are stable compounds of divalent carbon. Being strongly stabilized by pi-donating substituents, NHCs are good σ-donors but π-bonding with the metal is weak. For this reason, the bond between the carbon and the metal center is often represented by a single dative bond, whereas Fischer and Schrock carbenes are usually depicted with double bonds to metal. Continuing with this analogy, NHCs are often compared with well-established phosphine
-based ligands. Like phosphines, NHCs serve spectator ligands that influence catalysis through a combination of electronic and steric effects, but they do not directly bind substrates. Carbenes without a metal ligand have been produced in the lab, promising to reduce costs as required bonds to precious metals are no longer necessary.
. A variety of related reactions are used to interconvert light alkenes, e.g. butenes, propylene, and ethylene. Carbene-complexes are invoked as intermediates in the Fischer-Tropsch route to hydrocarbons. A variety of soluble carbene reagents, especially the Grubbs'
and molybdenum-imido catalysts have been applied to laboratory-scale synthesis
of natural product
s and materials science
.
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
. Carbene complexes for almost all transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s have been reported. Many methods for synthesizing them and reactions utilizing them have been reported. The term carbene ligand is a formalism since many are not derived from carbenes and almost none exhibit the reactivity characteristic of carbenes. Described often as M=CR2, they represent a class of organic ligands intermediate between alkyls (-CR3) and carbynes (≡CR). They feature in many catalytic reactions in the petrochemical industry and are of increasing interest in fine chemicals.
The characterization of (CO)5Cr(COCH3(Ph)) in the 1960s is often cited as the starting point of the area,
although carbenoid ligands had been previously implicated.
Classification of carbene complexes
Metal carbene complexes are often classified into two types. The Fischer carbenes named after Ernst Otto FischerErnst Otto Fischer
Ernst Otto Fischer was a German chemist who won the Nobel Prize for pioneering work in the area of organometallic chemistry.-Early life:...
feature strong π-acceptors at the metal and being electrophilic
Electrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
at the carbene carbon atom. Schrock carbenes, named after Richard R. Schrock
Richard R. Schrock
Richard Royce Schrock is an American chemist and Nobel laureate recognized for his contributions to the metathesis reaction used in organic chemistry.-Biography:...
, are characterized by more nucleophilic carbene carbon centers, these species typically feature higher valent metals. N-heterocyclic carbenes (NHCs) were popularlized following Arduengo's isolation of a stable free carbene in 1991. Reflecting the growth of the area, carbene complexes are now known with a broad range of different reactivities and diverse substituents. Often it is not possible to classify a carbene complex with regards to its electrophilicity or nucleophilicity.
Fischer carbenes
Fischer carbenes are found with:- low oxidation stateOxidation stateIn chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
metals - middle and late transition metals Fe(0)IronIron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
, Mo(0)MolybdenumMolybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores...
, Cr(0)ChromiumChromium is a chemical element which has the symbol Cr and atomic number 24. It is the first element in Group 6. It is a steely-gray, lustrous, hard metal that takes a high polish and has a high melting point. It is also odorless, tasteless, and malleable... - pi electron acceptor metal ligandLigandIn coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s - pi-donor substituentSubstituentIn organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s on methyleneMethyleneMethylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
group such as alkoxy and alkylated amino groups
The chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
ing (scheme 1) is based on electron δ-type
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
donation group of the filled methylene lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
orbital to an empty metal d-orbital, and pi electron back bonding of a filled metal d-orbital to the empty p-orbital on carbon. An example is the complex (CO)5Cr=C(NR2)Ph.
Fischer carbenes can be likened to ketones, with the carbene carbon being electrophilic, much like the carbonyl carbon of a ketone. Like ketones, Fischer carbene species can undergo Aldol
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
-like reactions. The hydrogen atoms attached to the carbon α to the carbene carbon are acidic, and can be deprotonated by a base such as n-butyllithium
N-Butyllithium
n-Butyllithium is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene...
, to give a nucleophile which can undergo further reaction.
This carbene is also the starting material for other reactions such as the Wulff-Dötz reaction. These type of carbenes were discovered by E. O. Fischer, and together with other achievements in organometalic chemistry, he was awarded the Nobel prize.
Schrock carbenes
Schrock carbenes do not have π-accepting ligands. These complexes are nucleophilicNucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
. Schrock carbenes are typically found with:
- high oxidation stateOxidation stateIn chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
s - early transition metals Ti(IV)TitaniumTitanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
, Ta(V)TantalumTantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as tantalium, the name comes from Tantalus, a character in Greek mythology. Tantalum is a rare, hard, blue-gray, lustrous transition metal that is highly corrosion resistant. It is part of the refractory... - pi-donor ligands
- hydrogen and alkyl substituents on carbenoid carbon
Bonding in such complexes can be viewed as the coupling of a triplet state metal and triplet carbene. These bonds are polarized towards carbon and therefore the methylene group is a nucleophile. An example of a Schrock carbene is the compound Ta(=C(H)But)(CH2But)3, with a tantalum(V) center doubly bonded to a neopentylidene ligand as well as three neopentyl ligands. An example of interest in organic synthesis is Tebbe's reagent
Tebbe's reagent
The Tebbe reagent is the organometallic compound with the formula 2TiCH2ClAl2. It used in the methylenation of carbonyl compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative...
.

N-heterocyclic carbenes
N-heterocyclic carbenes (NHCs) are generally derived from persistent carbenePersistent carbene
A persistent carbene is a type of carbene demonstrating particular stability. The best-known examples are diaminocarbenes with the general formula 2C:, where the 'R's are various functional groups...
s, which are stable compounds of divalent carbon. Being strongly stabilized by pi-donating substituents, NHCs are good σ-donors but π-bonding with the metal is weak. For this reason, the bond between the carbon and the metal center is often represented by a single dative bond, whereas Fischer and Schrock carbenes are usually depicted with double bonds to metal. Continuing with this analogy, NHCs are often compared with well-established phosphine
Phosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
-based ligands. Like phosphines, NHCs serve spectator ligands that influence catalysis through a combination of electronic and steric effects, but they do not directly bind substrates. Carbenes without a metal ligand have been produced in the lab, promising to reduce costs as required bonds to precious metals are no longer necessary.
Applications of carbene complexes
The main applications of metal carbenes involves none of the above classes of compounds, but rather heterogeneous catalysts used for alkene metathesis in the Shell higher olefin processShell higher olefin process
The Shell higher olefin process is a chemical process for the production of linear alpha olefins via ethylene oligomerization and olefin metathesis invented and exploited by Royal Dutch Shell. The olefin products are converted to fatty aldehydes and then to fatty alcohols, which are precursors...
. A variety of related reactions are used to interconvert light alkenes, e.g. butenes, propylene, and ethylene. Carbene-complexes are invoked as intermediates in the Fischer-Tropsch route to hydrocarbons. A variety of soluble carbene reagents, especially the Grubbs'
Grubbs' catalyst
Grubbs' Catalyst is a transition metal carbene complex named after Robert H. Grubbs, the chemist who first synthesized it. There are two generations of the catalyst, as shown on the right. In contrast to other olefin metathesis catalysts, Grubbs' Catalysts tolerate other functional groups in the...
and molybdenum-imido catalysts have been applied to laboratory-scale synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
of natural product
Natural product
A natural product is a chemical compound or substance produced by a living organism - found in nature that usually has a pharmacological or biological activity for use in pharmaceutical drug discovery and drug design...
s and materials science
Materials science
Materials science is an interdisciplinary field applying the properties of matter to various areas of science and engineering. This scientific field investigates the relationship between the structure of materials at atomic or molecular scales and their macroscopic properties. It incorporates...
.

