
Tetrathionate
Encyclopedia
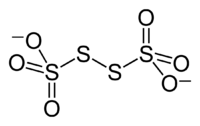
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
, S4O62−, is a sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
oxoanion derived from the compound tetrathionic acid, H2S4O6. Two of the sulfur atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s present in the ion are in oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
0 and two are in oxidation state +5. Alternatively, the compound can be viewed as the adduct
Adduct
An adduct is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is considered a distinct molecular species...
resulting from the binding of the Lewis base S22−
Disulfide
In chemistry, a disulfide usually refers to the structural unit composed of a linked pair of sulfur atoms. Disulfide usually refer to a chemical compound that contains a disulfide bond, such as diphenyl disulfide, C6H5S-SC6H5....
to SO3
Sulfur trioxide
Sulfur trioxide is the chemical compound with the formula SO3. In the gaseous form, this species is a significant pollutant, being the primary agent in acid rain. It is prepared on massive scales as a precursor to sulfuric acid.-Structure and bonding:Gaseous SO3 is a trigonal planar molecule of...
. Tetrathionate is one of the polythionates, a family of anions with the formula [Sn(SO3)2]2−. Its IUPAC name is (sulfonatodisulfanyl)sulfonate, the name of its corresponding acid is (sulfodisulfanyl)sulfonic acid. The Chemical Abstracts Service identifies tetrathionate by the CAS Number 15536-54-6.
Tetrathionate is a product of the oxidation
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
of thiosulfate
Thiosulfate
Thiosulfate is an oxyanion of sulfur. The prefix thio indicates that thiosulfate ion is a sulfate ion with one oxygen replaced by a sulfur. Thiosulfate occurs naturally and is produced by certain biochemical processes...
, S2O32−, by iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
, I2:
- 2S2O32− + I2 → S4O62− + 2I−IodideAn iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. This page is for the iodide ion and its salts. For information on organoiodides, see organohalides. In everyday life, iodide is most commonly encountered as a component of iodized salt,...
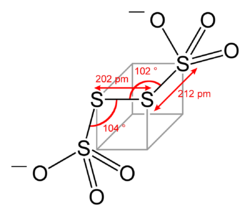
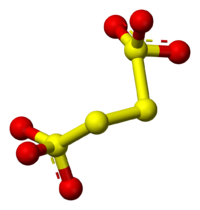
Structure
Tetrathionate's structure can be visualized by following three edges of a cube, as in the diagram below. The structure shown is the configuration of S4O62− in BaS4O6·2H2O and Na2S4O6·2H2O. Dihedral S-S-S-S angles approaching 90° are common in polysulfidePolysulfide
Polysulfides are a class of chemical compounds containing chains of sulfur atoms. There are two main classes of polysulfides: anions and organic polysulfides. Anions have the general formula Sn2−. These anions are the conjugate bases of the hydrogen polysulfides H2nSn...
s.
Compounds
Compounds containing the tetrathionate anion include sodiumSodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
tetrathionate, Na2S4O6, potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
tetrathionate, K2S4O6, and barium
Barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. Barium is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with...
tetrathionate dihydrate
Hydrate
Hydrate is a term used in inorganic chemistry and organic chemistry to indicate that a substance contains water. The chemical state of the water varies widely between hydrates, some of which were so labeled before their chemical structure was understood....
, BaS4O6·2H2O.
Properties
As other reduced species of sulfur, such as thiosulfate, tetrathionate can be responsible for the pitting corrosionPitting corrosion
Pitting corrosion, or pitting, is a form of extremely localized corrosion that leads to the creation of small holes in the metal. The driving power for pitting corrosion is the depassivation of a small area, which becomes anodic while an unknown but potentially vast area becomes cathodic, leading...
of carbon steel and stainless steel
Stainless steel
In metallurgy, stainless steel, also known as inox steel or inox from French "inoxydable", is defined as a steel alloy with a minimum of 10.5 or 11% chromium content by mass....
.

