Silyl hydride
Encyclopedia
Silicon hydrides are chemical compounds which contain a silicon–hydrogen bond. The silicon-to-hydrogen bond is longer than the C–H bond (148 compared to 105 pm) and weaker (299 compared to 338 kJ/mol). Hydrogen is more electronegative than silicon hence the naming convention of silyl hydrides. The parent compound SiH4 is called silane
, and an example is phenylsilane
. Silyl hydrides are very reactive and used as reducing agent
for example PMHS
.
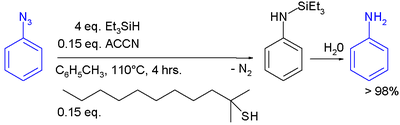
In one study triethylsilylhydride is used in the conversion of an phenyl azide
to an aniline
:
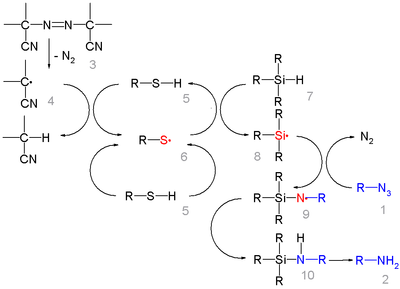
In this reaction ACCN is a radical initiator
and an aliphatic thiol
transfers radical character to the silylhydride. The triethylsilyl free radical then reacts with the azide with expulsion of nitrogen to a N-silylarylaminyl radical which grabs a proton from a thiol completing the catalytic cycle
:
Aqueous workup then gives aniline.
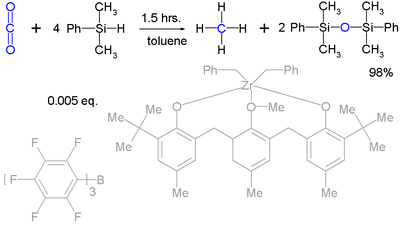
Silyl hydrides can even take up the reduction of robust molecules such as carbon dioxide
(to methane
) :
Although it takes a very complex catalyst system.
s, alkyne
s, imine
s, carbonyl
s and oxime
s to new organosilicon compounds in hydrosilylation. In the reaction of triphenylsilyl hydride with phenylacetylene
the reaction product is a trans or cis or the geminal
vinyl silane, for example :
In the related silylmetalation, a metal replaces the hydrogen atom.
Silane
Silane is a toxic, extremely flammable chemical compound with chemical formula SiH4. In 1857, the German chemists and Friedrich Woehler discovered silane among the products formed by the action of hydrochloric acid on aluminum silicide, which they had previously prepared...
, and an example is phenylsilane
Phenylsilane
Phenylsilane, also known as silylbenzene, a colorless liquid, is one of the simplest organosilanes with the formula C6H5SiH3. It is structurally related to toluene, with a silyl group replacing the methyl group. Both of these compounds have similar densities and boiling points due to these...
. Silyl hydrides are very reactive and used as reducing agent
Reductions with hydrosilanes
Reductions with hydrosilanes are chemical reactions that involve the combination of an organosilane with an organic substrate containing unsaturated or electron-withdrawing functionality...
for example PMHS
PMHS
Polymethylhydrosiloxane is a polymer with the general structure --. It is used in organic chemistry as a mild and stable reducing agent easily transferring hydrides to metal centers....
.
In one study triethylsilylhydride is used in the conversion of an phenyl azide
Phenyl azide
Phenylazide is an organic compound with the formula C6H5N3. It is one of the prototypical organic azides. It has a pungent odor. The structure consists of a linear azide substituent bound to a phenyl group...
to an aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
:
In this reaction ACCN is a radical initiator
Radical initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions . These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical initiators are utilized in industrial processes such...
and an aliphatic thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
transfers radical character to the silylhydride. The triethylsilyl free radical then reacts with the azide with expulsion of nitrogen to a N-silylarylaminyl radical which grabs a proton from a thiol completing the catalytic cycle
Catalytic cycle
A catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
:
Aqueous workup then gives aniline.
Silyl hydrides can even take up the reduction of robust molecules such as carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
(to methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
) :
Although it takes a very complex catalyst system.
Hydrosilylation
Silyl hydrides react with various unsaturated substrates such as alkeneAlkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s, alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
s, imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
s, carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
s and oxime
Oxime
An oxime is a chemical compound belonging to the imines, with the general formula R1R2C=NOH, where R1 is an organic side chain and R2 may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds...
s to new organosilicon compounds in hydrosilylation. In the reaction of triphenylsilyl hydride with phenylacetylene
Phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.-Preparation:...
the reaction product is a trans or cis or the geminal
Geminal
In chemistry, the term geminal refers to the relationship between two functional groups that are attached to the same atom...
vinyl silane, for example :
In the related silylmetalation, a metal replaces the hydrogen atom.