
Pyruvate formate lyase
Encyclopedia
Pyruvate formate lyase (PFL) is an enzyme
found in Escherichia coli
and other organism
s. It helps regulate anaerobic
glucose
metabolism
. Using radical non-redox chemistry, it catalyzes
the reversible conversion of pyruvate and coenzyme-A into formate
and acetyl-CoA
. The reaction occurs as follows:

. It has a 10-stranded beta/alpha barrel motif
into which is inserted a beta finger that contains major catalytic residues
. The active site
of the enzyme, elucidated by x-ray crystallography
, holds three essential amino acids that perform catalysis (Gly734
, Cys418
, and Cys419), three major residues that hold the substrate pyruvate close by (Arg435
, Arg176, and Ala272
), and two flanking hydrophobic residues (Trp333
and Phe432
).[1]
Studies have found structural similarities between the active site of pyruvate formate lyase and that of Class I and Class III ribonucleotide reductase
(RNR) enzymes.[1,4]
From Sources[2,6]
Note that each step is reversible.[2,6]
From Sources[2,6]
PFL activase is part of the radical SAM (S-adenosylmethionine) superfamily. The enzyme turns pyruvate formate lyase “on” by converting Gly734 (G-H) into a Gly734 radical (G*) via a 5'-deoxyadenosyl radical (radical SAM).[3]
For more information about radical SAM activation and radical SAM enzymes, see the discussion by Wang et al., 2007.[7]
PFL deactivase (DA) turns pyruvate formate lyase “off” by quenching the Gly734 radical.[5] Furthermore, pyruvate formate lyase is sensitive to molecular oxygen
(O2), the presence of which shuts the enzyme off.[8]
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
found in Escherichia coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
and other organism
Organism
In biology, an organism is any contiguous living system . In at least some form, all organisms are capable of response to stimuli, reproduction, growth and development, and maintenance of homoeostasis as a stable whole.An organism may either be unicellular or, as in the case of humans, comprise...
s. It helps regulate anaerobic
Anaerobic respiration
Anaerobic respiration is a form of respiration using electron acceptors other than oxygen. Although oxygen is not used as the final electron acceptor, the process still uses a respiratory electron transport chain; it is respiration without oxygen...
glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
metabolism
Metabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
. Using radical non-redox chemistry, it catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the reversible conversion of pyruvate and coenzyme-A into formate
Formate
Formate or methanoate is the ion CHOO− or HCOO− . It is the simplest carboxylate anion. It is produced in large amounts in the hepatic mitochondria of embryonic cells and in cancer cells by the folate cycle Formate or methanoate is the ion CHOO− or HCOO− (formic acid minus one hydrogen ion). It...
and acetyl-CoA
Acetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
. The reaction occurs as follows:

Structure
Pyruvate formate lyase is a homodimer made of 85 kDa, 759-residue subunitsAmino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
. It has a 10-stranded beta/alpha barrel motif
Beta barrel
A beta barrel is a large beta-sheet that twists and coils to form a closed structure in which the first strand is hydrogen bonded to the last.Beta-strands in beta-barrels are typically arranged in an antiparallel fashion...
into which is inserted a beta finger that contains major catalytic residues
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
. The active site
Active site
In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that...
of the enzyme, elucidated by x-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
, holds three essential amino acids that perform catalysis (Gly734
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
, Cys418
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
, and Cys419), three major residues that hold the substrate pyruvate close by (Arg435
Arginine
Arginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during...
, Arg176, and Ala272
Alanine
Alanine is an α-amino acid with the chemical formula CH3CHCOOH. The L-isomer is one of the 20 amino acids encoded by the genetic code. Its codons are GCU, GCC, GCA, and GCG. It is classified as a nonpolar amino acid...
), and two flanking hydrophobic residues (Trp333
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
and Phe432
Phenylalanine
Phenylalanine is an α-amino acid with the formula C6H5CH2CHCOOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form...
).[1]
Studies have found structural similarities between the active site of pyruvate formate lyase and that of Class I and Class III ribonucleotide reductase
Ribonucleotide reductase
Ribonucleotide reductase is an enzyme that catalyzes the formation of deoxyribonucleotides from ribonucleotides. Deoxyribonucleotides in turn are used in the synthesis of DNA. The reaction catalyzed by RNR is strictly conserved in all living organisms...
(RNR) enzymes.[1,4]
Roles of the three catalytic residues
- Gly734 (glycyl radical) – transfers the radical on and off Cys418, via Cys419
- Cys418 (thiyl radical) – does acylationAcylationIn chemistry, acylation is the process of adding an acyl group to a compound. The compound providing the acyl group is called the acylating agent....
chemistry on the carbon atom of the pyruvate carbonylCarbonylIn organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups.... - Cys419 (thiyl radical) – performs hydrogen-atom transfers
From Sources[2,6]
Steps
- The proposed mechanism for pyruvate formate lyase begins with radical transfer from Gly734 to Cys418, via Cys419.
- The Cys418 thiyl radical adds covalently to C2 (second carbon atom) of pyruvate, generating an acetyl-enzyme intermediate (which now contains the radical).
- The acetyl-enzyme intermediate releases a formyl radical that undergoes hydrogen-atom transfer with Cys419. This generates formate and a Cys419 radical.
- coenzyme-A comes in and undergoes hydrogen-atom transfer with the Cys419 radical to generate a coenzyme-A radical.
- The coenzyme-A radical then picks up the acetyl group from Cys418 to generate acetyl-CoA, leaving behind a Cys418 radical.
- Pyruvate formate lyase can then undergo radical transfer to put the radical back onto Gly734.
Note that each step is reversible.[2,6]
From Sources[2,6]
Regulation
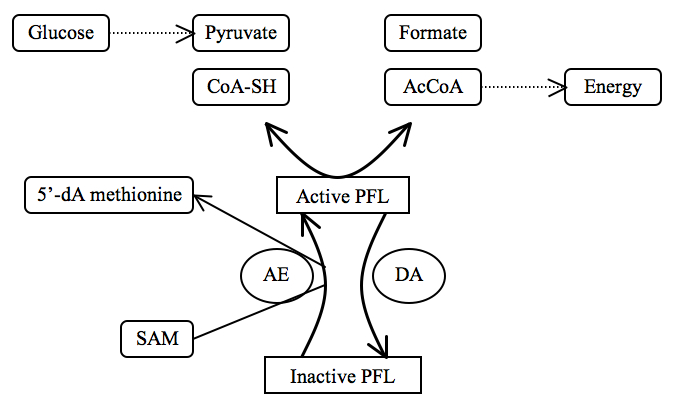
Two additional enzymes regulate the “on” and “off” states of pyruvate formate lyase to regulate anaerobic glucose metabolism: PFL activase (AE) and PFL deactivase (DA). Activated pyruvate formate lyase allows formation of acetyl-CoA, a small molecule important in the production of energy, when pyruvate is available. Deactivated pyruvate formate lyase, even with substrates present, does not catalyze the reaction.
PFL activase is part of the radical SAM (S-adenosylmethionine) superfamily. The enzyme turns pyruvate formate lyase “on” by converting Gly734 (G-H) into a Gly734 radical (G*) via a 5'-deoxyadenosyl radical (radical SAM).[3]
For more information about radical SAM activation and radical SAM enzymes, see the discussion by Wang et al., 2007.[7]
PFL deactivase (DA) turns pyruvate formate lyase “off” by quenching the Gly734 radical.[5] Furthermore, pyruvate formate lyase is sensitive to molecular oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
(O2), the presence of which shuts the enzyme off.[8]

