
Beta barrel
Encyclopedia
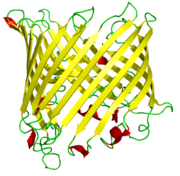

Beta-strands in beta-barrels are typically arranged in an antiparallel
Antiparallel (biochemistry)
In biochemistry, two molecules are antiparallel if they run side-by-side in opposite directions or when both strands are complimentary to each other....
fashion. Barrel structures are commonly found in porins
Porin (protein)
Porins are beta barrel proteins that cross a cellular membrane and act as a pore through which molecules can diffuse. Unlike other membrane transport proteins, porins are large enough to allow passive diffusion, i.e., they act as channels that are specific to different types of molecules...
and other proteins that span cell membrane
Cell membrane
The cell membrane or plasma membrane is a biological membrane that separates the interior of all cells from the outside environment. The cell membrane is selectively permeable to ions and organic molecules and controls the movement of substances in and out of cells. It basically protects the cell...
s and in proteins that bind hydrophobic ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s in the barrel center, as in lipocalins. Porin-like barrel structures are encoded by as many as 2–3% of the genes in Gram-negative
Gram-negative
Gram-negative bacteria are bacteria that do not retain crystal violet dye in the Gram staining protocol. In a Gram stain test, a counterstain is added after the crystal violet, coloring all Gram-negative bacteria with a red or pink color...
bacteria.
In many cases the strands contain alternating polar
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
and hydrophobic amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s, so that the hydrophobic residues are oriented into the interior of the barrel to form a hydrophobic core and the polar residues are oriented toward the outside of the barrel on the solvent-exposed surface. Porins and other membrane protein
Membrane protein
A membrane protein is a protein molecule that is attached to, or associated with the membrane of a cell or an organelle. More than half of all proteins interact with membranes.-Function:...
s containing beta barrels reverse this pattern, with hydrophobic residues oriented toward the exterior where they contact the surrounding lipid
Lipid
Lipids constitute a broad group of naturally occurring molecules that include fats, waxes, sterols, fat-soluble vitamins , monoglycerides, diglycerides, triglycerides, phospholipids, and others...
s, and hydrophilic residues oriented toward the interior pore.
All beta-barrels can be classified in terms of two integer parameters: the number of strands in the beta-sheet, n, and the "shear number", S, a measure of the stagger of the strands in the beta-sheet.
These two parameters (n and S) are related to the inclination angle of the beta strands relative to the axis of the barrel.
Up-and-down beta barrel
Up-and-down barrels are the simplest barrel topology and consist of a series of beta strands, each of which is hydrogen-bonded to the strands immediately before and after it in the primary sequence.Greek key
Greek key barrels have some beta strands adjacent in space that are not adjacent in sequence. Beta barrels generally consist of at least one Greek key structural motifStructural motif
In a chain-like biological molecule, such as a protein or nucleic acid, a structural motif is a supersecondary structure, which appears also in a variety of other molecules...
linked to a beta hairpin
Beta hairpin
The beta hairpin structural motif is the simplest protein motif involving two beta strands that look like a hairpin. The motif consists of two strands that are adjacent in primary structure oriented in an antiparallel arrangement and linked by a short loop of two to five amino acids...
, or two successive Greek keys.
Jelly roll
The jelly roll barrel, also known as the Swiss roll, is a complex nonlocal structure in which four pairs of antiparallel beta sheets, only one of which is adjacent in sequence, are "wrapped" in three dimensions to form a barrel shape.Porins
Sixteen- or eighteen-stranded beta barrel structures are common in porins, which function as transporters for ions and small molecules that cannot diffuseDiffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
across a cellular membrane. Such structures appear in the outer membrane
Outer membrane
The bacterial outer membrane is found in Gram-negative bacteria. Its composition is distinct from that of the cytoplasmic membrane - among other things, the outer leaflet of the membrane includes a complex lipopolysaccharide whose lipid portion acts as an endotoxin - and it is linked to the cell's...
s of gram-negative
Gram-negative
Gram-negative bacteria are bacteria that do not retain crystal violet dye in the Gram staining protocol. In a Gram stain test, a counterstain is added after the crystal violet, coloring all Gram-negative bacteria with a red or pink color...
bacteria, chloroplast
Chloroplast
Chloroplasts are organelles found in plant cells and other eukaryotic organisms that conduct photosynthesis. Chloroplasts capture light energy to conserve free energy in the form of ATP and reduce NADP to NADPH through a complex set of processes called photosynthesis.Chloroplasts are green...
s, and mitochondria. The central pore of the protein, sometimes known as the eyelet, is lined with charged residues arranged so that the positive and negative charges appear on opposite sides of the pore. A long loop between two beta sheets partially occludes the central channel; the exact size and conformation of the loop helps in discriminating between molecules passing through the transporter.
Lipocalins
Lipocalins are typically eight-stranded beta barrel proteins that are often secreted into the extracellular environment. Their most distinctive feature is their ability to bind and transport small hydrophobic molecules in a beta barrel calyxCalyx (zoology)
-Cnidarians:The spicules containing the basal portion of the upper tentacular part of the polyp of some soft corals .-Entoprocta:A body part of the Entoprocta from which tentacles arise and the mouth and anus are located.-Echinoderms:...
. Examples of the family include retinol binding protein
Retinol binding protein
Retinol-binding proteins are a family of proteins with diverse functions. They are carrier proteins that bind retinol.Assessment of retinol-binding protein is used to determine visceral protein mass in nutritional studies related to health.-Genes:...
s (RBPs) and major urinary proteins
Major urinary proteins
Major urinary proteins , also known as α2u-globulins, are a subfamily of proteins found in abundance in the urine and other secretions of many animals. Mups provide a small range of identifying information about the donor animal, when detected by the vomeronasal organ of the receiving animal. They...
(Mups). RBP binds and transports retinol
Retinol
Retinol is one of the animal forms of vitamin A. It is a diterpenoid and an alcohol. It is convertible to other forms of vitamin A, and the retinyl ester derivative of the alcohol serves as the storage form of the vitamin in animals....
(vitamin A), while Mups bind a number of small, organic pheromones, including 2-sec-butyl-4,5-dihydrothiazole (abbreviated as SBT or DHT), 6-hydroxy-6-methyl-3-heptanone (HMH) and 2,3 dihydro-exo-brevicomin (DHB).
Shear number
A piece of paper can be formed into a cylinder by bringing opposite sides together. The two edges come together to form a line. Shear can be created by sliding the two edges parallel to that line. Likewise, a beta barrel can be formed by bringing the edges of a beta sheet together to form a cylinder. If those edges are displaced, then shear will be created.A similar definition of shear is found in geology, where shear refers to a displacement within rock perpendicular to the surface of the rock. In physics, the amount of displacement is referred to as shear strain, which has units of length. Shear number is a measure of shear strain in which the displacement is measured in units of "amino acid residues".
The determination of shear number requires the assumption that each amino acid in one strand of a beta sheet is adjacent to just one amino acid in the neighboring strand. (This assumption may not hold if, for example, a beta bulge
Beta bulge
A beta bulge is a localized disruption of the regular hydrogen bonding of a beta sheet, usually by inserting a residue with helical dihedral angles into one or both H-bonded β-strands.-Types:...
is present.
) To illustrate, S will be calculated for green fluorescent protein
Green fluorescent protein
The green fluorescent protein is a protein composed of 238 amino acid residues that exhibits bright green fluorescence when exposed to blue light. Although many other marine organisms have similar green fluorescent proteins, GFP traditionally refers to the protein first isolated from the...
. This protein was chosen because the beta barrel contains both parallel and antiparallel strands. The particular example used, , is one of the few structures of this protein that is not obtained from a mutant protein.
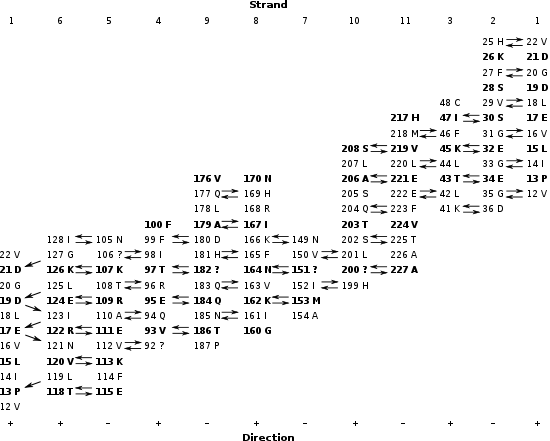
From the last figure, the order of strands in the barrel is found to be: 1 6 5 4 9 8 7 10 11 3 2.
To determine which amino acid residues are adjacent in the beta strands, the location of hydrogen bonds is determined. The figure below shows the calculated positions of hydrogen bonds. The residues are labeled with a residue number and a one-letter amino acid code (the label is placed near the alpha carbon). Only the backbone atoms of the beta barrel are shown, and, of that, only the front slab is shown. It appears that, for example, residues 31 G, on strand 2, 16 V on strand 1, and 121 N on strand 6 are adjacent.

Dynamical features
Beta-barrels in proteins may carry out low-frequency breathing-like motion as observed by the Raman spectroscopyRaman spectroscopy
Raman spectroscopy is a spectroscopic technique used to study vibrational, rotational, and other low-frequency modes in a system.It relies on inelastic scattering, or Raman scattering, of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range...
and analyzed with the quasi-continuum model.
For more about the low-frequency collective motions in biomacromolecules and its biological function, see low-frequency collective motion in proteins and DNA.
Further reading
- Branden C, Tooze J. (1999). Introduction to Protein Structure 2nd ed. Garland Publishing: New York, NY. ISBN 0815323042.
External links
- Explanation of all-beta topologies: "orthogonal beta-sandwiches" are beta-barrels (as defined in this article); "aligned" beta-sandwiches" correspond to beta-sandwich folds in SCOPSCOPSCOP may refer to:* Structural Classification of Proteins* Suprachiasmatic nucleus circadian oscillatory protein, a member of the leucine-rich repeat protein family* Société coopérative, a type of corporation in France...
classification. - all-beta folds in SCOP database (folds 54 to 100 are water-soluble beta-barrels).
- General classification and images of protein structures from Jane Richardson lab
- Images and examples of transmembrane beta-barrels
- Stockholm Bioinformatics Center review of transmembrane proteins
- The Lipocalin Website

