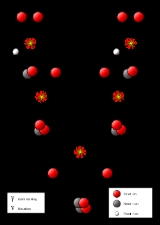
Proton-proton chain reaction
Encyclopedia

Nuclear fusion
Nuclear fusion is the process by which two or more atomic nuclei join together, or "fuse", to form a single heavier nucleus. This is usually accompanied by the release or absorption of large quantities of energy...
reactions by which star
Star
A star is a massive, luminous sphere of plasma held together by gravity. At the end of its lifetime, a star can also contain a proportion of degenerate matter. The nearest star to Earth is the Sun, which is the source of most of the energy on Earth...
s convert hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
to helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
, the primary alternative being the CNO cycle
CNO cycle
The CNO cycle is one of two sets of fusion reactions by which stars convert hydrogen to helium, the other being the proton–proton chain. Unlike the proton–proton chain reaction, the CNO cycle is a catalytic cycle. Theoretical models show that the CNO cycle is the dominant source of energy in stars...
. The proton–proton chain dominates in stars the size of the Sun
Sun
The Sun is the star at the center of the Solar System. It is almost perfectly spherical and consists of hot plasma interwoven with magnetic fields...
or smaller.
In general, proton–proton fusion can occur only if the temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
(i.e. kinetic energy
Kinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
) of the protons is high enough to overcome their mutual electrostatic or Coulomb repulsion.
In the Sun, deuterium-producing events are rare enough (the vast majority of these events produces a diproton
Diproton
A diproton is a hypothetical isotope of helium nucleus consisting of two protons and no neutrons, and is predicted to be less stable than 5He...
instead) that a complete conversion of its hydrogen would take more than (ten billion) years at the prevailing conditions of its core. The fact that the Sun is still shining is due to the slow nature of this reaction; if it went more quickly, the Sun would have exhausted its hydrogen long ago.
History of the Proton-Proton Chain Reaction
The theory that proton–proton reactions were the basic principle by which the Sun and other stars burn was advocated by Arthur Stanley EddingtonArthur Stanley Eddington
Sir Arthur Stanley Eddington, OM, FRS was a British astrophysicist of the early 20th century. He was also a philosopher of science and a popularizer of science...
in the 1920s. At the time, the temperature of the Sun was considered too low to overcome the Coulomb barrier
Coulomb barrier
The Coulomb barrier, named after Coulomb's law, which is named after physicist Charles-Augustin de Coulomb , is the energy barrier due to electrostatic interaction that two nuclei need to overcome so they can get close enough to undergo a nuclear reaction...
. After the development of quantum mechanics
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
, it was discovered that tunneling
Quantum tunnelling
Quantum tunnelling refers to the quantum mechanical phenomenon where a particle tunnels through a barrier that it classically could not surmount. This plays an essential role in several physical phenomena, such as the nuclear fusion that occurs in main sequence stars like the sun, and has important...
of the wavefunction
Wavefunction
Not to be confused with the related concept of the Wave equationA wave function or wavefunction is a probability amplitude in quantum mechanics describing the quantum state of a particle and how it behaves. Typically, its values are complex numbers and, for a single particle, it is a function of...
s of the protons through the repulsive barrier allows for fusion at a lower temperature than the classical
Classical physics
What "classical physics" refers to depends on the context. When discussing special relativity, it refers to the Newtonian physics which preceded relativity, i.e. the branches of physics based on principles developed before the rise of relativity and quantum mechanics...
prediction.
Even so, it was unclear how proton-proton fusion might proceed, because the most obvious product, helium-2 (diproton
Diproton
A diproton is a hypothetical isotope of helium nucleus consisting of two protons and no neutrons, and is predicted to be less stable than 5He...
), is unstable and immediately dissociates back into a pair of protons. In 1939, Hans Bethe
Hans Bethe
Hans Albrecht Bethe was a German-American nuclear physicist, and Nobel laureate in physics for his work on the theory of stellar nucleosynthesis. A versatile theoretical physicist, Bethe also made important contributions to quantum electrodynamics, nuclear physics, solid-state physics and...
proposed that one of the protons could beta decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
into a neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
via the weak interaction
Weak interaction
Weak interaction , is one of the four fundamental forces of nature, alongside the strong nuclear force, electromagnetism, and gravity. It is responsible for the radioactive decay of subatomic particles and initiates the process known as hydrogen fusion in stars...
during the brief moment of fusion, making deuterium
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
the initial product in the chain. This idea was part of the body of work in stellar nucleosynthesis
Stellar nucleosynthesis
Stellar nucleosynthesis is the collective term for the nuclear reactions taking place in stars to build the nuclei of the elements heavier than hydrogen. Some small quantity of these reactions also occur on the stellar surface under various circumstances...
for which Bethe won the 1967 Nobel Prize in Physics
Nobel Prize in Physics
The Nobel Prize in Physics is awarded once a year by the Royal Swedish Academy of Sciences. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895 and awarded since 1901; the others are the Nobel Prize in Chemistry, Nobel Prize in Literature, Nobel Peace Prize, and...
.
The Proton-Proton Chain Reaction
The first step involves the fusion of two hydrogen nuclei (protonProton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s) into deuterium
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
, releasing a positron
Positron
The positron or antielectron is the antiparticle or the antimatter counterpart of the electron. The positron has an electric charge of +1e, a spin of ½, and has the same mass as an electron...
and a neutrino
Neutrino
A neutrino is an electrically neutral, weakly interacting elementary subatomic particle with a half-integer spin, chirality and a disputed but small non-zero mass. It is able to pass through ordinary matter almost unaffected...
as one proton changes into a neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
.
- {| border="0"
|- style="height:2em;"
| ||+ || ||→ || ||+ || ||+ || ||+ ||
|}
This first step is extremely slow, both because the protons have to tunnel
Quantum tunnelling
Quantum tunnelling refers to the quantum mechanical phenomenon where a particle tunnels through a barrier that it classically could not surmount. This plays an essential role in several physical phenomena, such as the nuclear fusion that occurs in main sequence stars like the sun, and has important...
through the Coulomb barrier and because it depends on weak interaction
Weak interaction
Weak interaction , is one of the four fundamental forces of nature, alongside the strong nuclear force, electromagnetism, and gravity. It is responsible for the radioactive decay of subatomic particles and initiates the process known as hydrogen fusion in stars...
s.
The positron immediately annihilates
Electron-positron annihilation
Electron–positron annihilation occurs when an electron and a positron collide. The result of the collision is the annihilation of the electron and positron, and the creation of gamma ray photons or, at higher energies, other particles:...
with an electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
, and their mass energy, as well as their kinetic energy, is carried off by two gamma ray
Gamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
s.
- {| border="0"
|- style="height:2em;"
| ||+ || ||→ ||2 ||+ ||
|}
After this, the deuterium produced in the first stage can fuse with another hydrogen to produce a light isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
of helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
, :
- {| border="0"
|- style="height:2em;"
| ||+ || ||→ || ||+ || ||+ ||
|}
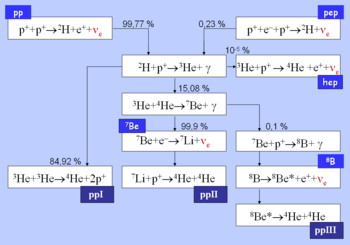
From here there are three possible paths to generate helium isotope . In pp I helium-4 comes from fusing two of the helium-3 nuclei produced; the pp II and pp III branches fuse with a pre-existing to make beryllium
Beryllium
Beryllium is the chemical element with the symbol Be and atomic number 4. It is a divalent element which occurs naturally only in combination with other elements in minerals. Notable gemstones which contain beryllium include beryl and chrysoberyl...
. In the Sun, branch pp I takes place with a frequency of 86%, pp II with 14% and pp III with 0.11%. There is also an extremely rare pp IV branch.
The pp I branch
- {| border="0"
|- style="height:2em;"
| ||+ || ||→ || ||+ ||2 ||+ ||
|}
The complete pp I chain reaction releases a net energy of .
The pp I branch is dominant at temperatures of 10 to .
Below , the PP chain does not produce much .
The pp II branch
- {| border="0"
|- style="height:2em;"
| ||+ || ||→ || ||+ ||
|- style="height:2em;"
| ||+ || ||→ || ||+ || ||+ || ||/ ||
|- style="height:2em;"
| ||+ || ||→ ||2
|}
The pp II branch is dominant at temperatures of 14 to .
90% of the neutrinos produced in the reaction * carry an energy of , while the remaining 10% carry (depending on whether lithium-7 is in the ground state or an excited state, respectively).
The pp III branch
- {| border="0"
|- style="height:2em;"
| ||+ || ||→ || ||+ ||
|- style="height:2em;"
| ||+ || ||→ || ||+ ||
|- style="height:2em;"
| || || ||→ || ||+ || ||+ || ||+ ||
|- style="height:2em;"
| || || ||→ ||2
|}
The pp III chain is dominant if the temperature exceeds .
The pp III chain is not a major source of energy in the Sun (only 0.11%), but was very important in the solar neutrino problem
Solar neutrino problem
The solar neutrino problem was a major discrepancy between measurements of the numbers of neutrinos flowing through the Earth and theoretical models of the solar interior, lasting from the mid-1960s to about 2002...
because it generates very high energy neutrinos (up to ).
The pp IV or Hep
This reaction is predicted but has never been observed due to its great rarity (about in the Sun). In this reaction, Helium-3 reacts directly with a proton to give helium-4, with an even higher possible neutrino energy (up to 18.8 MeV).- {| border="0"
|- style="height:2em;"
| ||+ || ||→ || ||+ || ||+ || ||+ ||
|}
Energy release
Comparing the mass of the final helium-4 atom with the masses of the four protons reveals that 0.007 or 0.7% of the mass of the original protons has been lost. This mass has been converted into energy, in the form of gamma rays and neutrinos released during each of the individual reactions. The total energy yield of one whole chain is .Only energy released as gamma rays will interact with electrons and protons and heat the interior of the Sun. This heating supports the Sun and prevents it from collapsing under its own weight.
Neutrinos do not interact significantly with matter and do not help support the Sun against gravitational collapse. The neutrinos in the ppI, ppII and ppIII chains carry away 2.0%, 4.0% and 28.3% of the energy in those reactions respectively.
The pep reaction
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
can also be produced by the rare pep (proton–electron–proton) reaction (electron capture
Electron capture
Electron capture is a process in which a proton-rich nuclide absorbs an inner atomic electron and simultaneously emits a neutrino...
):
- {| border="0"
|- style="height:2em;"
| ||+ || ||+ || ||→ || ||+ ||
|}
In the Sun, the frequency ratio of the pep reaction versus the pp reaction is 1:400. However, the neutrinos released by the pep reaction are far more energetic: while neutrinos produced in the first step of the pp reaction range in energy up to , the pep reaction produces sharp-energy-line neutrinos of .
Both the pep and pp reactions can be seen as two different Feynman
Feynman diagram
Feynman diagrams are a pictorial representation scheme for the mathematical expressions governing the behavior of subatomic particles, first developed by the Nobel Prize-winning American physicist Richard Feynman, and first introduced in 1948...
representations of the same basic interaction, where the electron passes to the right side of the reaction as an anti-electron. This is represented in the figure of proton–proton and electron-capture chain reactions in a star, available at the NDM'06 web site.

