
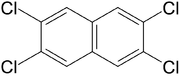
Polychlorinated naphthalene
Encyclopedia
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
with naphthalene
Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
, a soft, pungent solid made from coal or petroleum and often used for mothproofing. The generic chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
is C
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
10H
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
10-nCl
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
n. Commercial PCNs are mixtures of up to 75 chlorinated naphthalene congeners plus byproducts and are often described by the total fraction of chlorine. As the chlorine proportion grows, PCNs become increasingly waxy or firm solids at room temperature. Some PCNs make effective insulating coatings for electrical wires. Others have been used as wood preservatives, as rubber and plastic additives, for capacitor dielectrics and in lubricants.
PCNs started to be produced for high-volume uses around 1910 in both Europe and the United States. In Europe the largest volume products were called Nibren waxes, made in Germany by Bayer
Bayer
Bayer AG is a chemical and pharmaceutical company founded in Barmen , Germany in 1863. It is headquartered in Leverkusen, North Rhine-Westphalia, Germany and well known for its original brand of aspirin.-History:...
. Other European PCN tradenames included Seekay (UK, from ICI
Imperial Chemical Industries
Imperial Chemical Industries was a British chemical company, taken over by AkzoNobel, a Dutch conglomerate, one of the largest chemical producers in the world. In its heyday, ICI was the largest manufacturing company in the British Empire, and commonly regarded as a "bellwether of the British...
), Clonacire (France), Cerifal (Italy) and Woskol (Poland). In the United States, the largest volume PCN products were called Halowax, from a New York company of the same name that was later owned by Union Carbide
Union Carbide
Union Carbide Corporation is a wholly owned subsidiary of The Dow Chemical Company. It currently employs more than 2,400 people. Union Carbide primarily produces chemicals and polymers that undergo one or more further conversions by customers before reaching consumers. Some are high-volume...
and then taken over by Koppers
Koppers
Koppers is a global chemical and materials company based in Downtown Pittsburgh, Pennsylvania, United States in an art-deco 1920s skyscraper, the Koppers Tower.-Structure:...
of Pittsburgh, PA, now Beazer East. Although trace amounts of PCNs may be released by natural processes such as wildfires, their industrial uses increased the apparent rates of accumulation in the environment by factors of 10,000 or more.
After about twenty years of commercial production, health hazards began to be reported in workers exposed to PCNs: severe skin rashes and liver disease
Liver disease
Liver disease is a broad term describing any single number of diseases affecting the liver.-Diseases:* Hepatitis, inflammation of the liver, caused mainly by various viruses but also by some poisons , autoimmunity or hereditary conditions...
that led to deaths of workers. A conference about the hazards was organized at Harvard School of Public Health
Harvard School of Public Health
The Harvard School of Public Health is one of the professional graduate schools of Harvard University, located in the Longwood Area of the Boston, Massachusetts neighborhood of Mission Hill, which is next to Harvard Medical School. HSPH is considered a significant school focusing on health in the...
in 1937, and several more publications dealing with PCN hazards appeared before 1940. PCNs containing three or more chlorines per molecule have typically been found more hazardous than those with fewer, but as the maximum of eight is approached, hazards appear to decrease.
There was a lag of about forty years between disclosure of PCN hazards and government regulation. In the U.S. exposure to PCNs was drastically reduced after 1976, following enactment of the Toxic Substances Control Act
Toxic Substances Control Act
The Toxic Substances Control Act is a United States law, passed by the United States Congress in 1976, that regulates the introduction of new or already existing chemicals. It grandfathered most existing chemicals, in contrast to the Registration, Evaluation and Authorization of Chemicals ...
. Major equipment manufacturers banned PCNs in their products, and major PCN producers discontinued operations. By 1983 worldwide PCN production had almost halted except for small amounts used in testing and research. Until recent years duPont produced a synthetic rubber, Neoprene
Neoprene
Neoprene or polychloroprene is a family of synthetic rubbers that are produced by polymerization of chloroprene. Neoprene in general has good chemical stability, and maintains flexibility over a wide temperature range...
FB, made in Northern Ireland
Northern Ireland
Northern Ireland is one of the four countries of the United Kingdom. Situated in the north-east of the island of Ireland, it shares a border with the Republic of Ireland to the south and west...
using pentachloronaphthalene. Today PCNs are offered commercially by only a few companies, including Ukrgeochem of Simferopol, Ukraine.
While some PCNs can be broken down by sunlight and, at slow rates, by certain microorganisms, many PCNs persist in the environment. After more than 80 years of use and total production of several hundred million kilograms, PCN residues are widespread. Acute exposure causes chloracne
Chloracne
Chloracne is an acne-like eruption of blackheads, cysts, and pustules associated with over-exposure to certain halogenated aromatic compounds, such as chlorinated dioxins and dibenzofurans...
. Chronic exposure increases risk of liver disease. Increased cancer risks have been suspected but so far not shown. Current concerns about PCNs include their release as byproducts of waste incineration.

