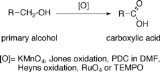
Oxidation of primary alcohols to carboxylic acids
Encyclopedia
The oxidation of primary alcohols to carboxylic acids is an important oxidation
reaction in organic chemistry
.
When a primary alcohol
is converted to a carboxylic acid
, the terminal carbon atom increases its oxidation state
by four. Oxidants able to perform this operation in complex organic molecules, featuring other oxidation-sensitive functional groups, must possess substantial selectivity. The most common oxidants are potassium permanganate
(KMnO4), Jones reagent, PDC
in DMF
, Heyns oxidation, ruthenium tetroxide
(RuO4) and TEMPO
.

, acetone
or t-BuOH. KMnO4 will readily react with a carbon - carbon double bond before oxidizing a primary alcohol.
Normally, these oxidations are performed under strong alkaline conditions using a ca. 1N NaOH or KOH
solution, because this promotes a greater oxidation speed and selectivity. In substrates sensitive to strong base, the reaction can be carried out at a lower pH – or even under acidic conditions — at the cost of a greatly decreased reaction velocity.
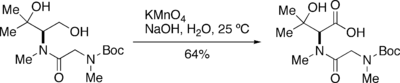 KMnO4 is decomposed in water, resulting in formation of manganese dioxide (MnO2) and gaseous oxygen. This decomposition is catalyzed by acid, base and MnO2. As the extent of this decomposition is difficult to estimate during the oxidation of primary alcohols, the quantity of KMnO4 must be adjusted during the oxidation by adding it sequentially until the oxidation is complete.
KMnO4 is decomposed in water, resulting in formation of manganese dioxide (MnO2) and gaseous oxygen. This decomposition is catalyzed by acid, base and MnO2. As the extent of this decomposition is difficult to estimate during the oxidation of primary alcohols, the quantity of KMnO4 must be adjusted during the oxidation by adding it sequentially until the oxidation is complete.
(CrO3) in aqueous sulfuric acid
, which results in formation of a reddish solution containing chromic acid (H2CrO4) and oligomers thereof. Addition of Jones reagent to a solution of a primary alcohol in acetone
— as first described by Jones —
results in oxidation of the alcohol to a carboxylic acid. This classical protocol, involving a direct addition, is used very often regardless of the fact that it frequently leads to the formation of substantial amounts of ester
s — possessing the structure R-CO-O-CH2-R — derived from oxidative dimerization of primary alcohols. Holland and Gilman proved that this side reaction can be greatly suppressed by following the inverse addition protocol whereby a solution of the primary alcohol in acetone is slowly added to Jones reagent under conditions as dilute as practical.
Jones reagent interacts with secondary alcohols resulting in oxidation to ketones. Treatment of compounds, containing both primary and secondary alcohols, with Jones reagent leads to formation of ketoacids.
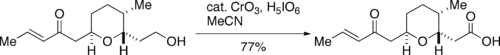
Problems encountered with the use of large quantities of chromium trioxide, which is toxic and dangerous for the environment, prompted the development by Zhao of a catalytic procedure, involving treatment with excess of periodic acid
(H5IO6) in presence of about 1.2 mol% of CrO3. Zhao's procedure for the use of catalytic CrO3 is very well-suited for reactions on a large scale.
.
The primary alcohol is oxidized to an aldehyde
using one of the many existing procedures (e.g. IBX oxidation, Dess-Martin-Periodinane). The aldehyde can then be subjected to the conditions of the Pinnick oxidation
using sodium chlorite
.
This sequence is often used in natural product synthesis, Nicolaou et al. applied it in their synthesis of Platencin.
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
reaction in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
.
When a primary alcohol
Primary alcohol
A primary alcohol is an alcohol which has the hydroxyl radical connected to a primary carbon. It can also be defined as a molecule containing a “–CH2OH” group.Examples include ethanol and butanol....
is converted to a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
, the terminal carbon atom increases its oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
by four. Oxidants able to perform this operation in complex organic molecules, featuring other oxidation-sensitive functional groups, must possess substantial selectivity. The most common oxidants are potassium permanganate
Potassium permanganate
Potassium permanganate is an inorganic chemical compound with the formula KMnO4. It is a salt consisting of K+ and MnO4− ions. Formerly known as permanganate of potash or Condy's crystals, it is a strong oxidizing agent. It dissolves in water to give intensely purple solutions, the...
(KMnO4), Jones reagent, PDC
Pyridinium chlorochromate
Pyridinium chlorochromate is a reddish orange solid reagent used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones. Pyridinium chlorochromate, or PCC, will not fully oxidize a primary alcohol to the carboxylic acid as does the Jones reagent. A disadvantage to using PCC is...
in DMF
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
, Heyns oxidation, ruthenium tetroxide
Ruthenium tetroxide
Ruthenium tetroxide is a diamagnetic tetrahedral ruthenium compound. As expected for a charge-neutral symmetrical oxide, it is quite volatile. The analogous OsO4 is more widely used and better known...
(RuO4) and TEMPO
TEMPO
oxyl, or oxidanyl or TEMPO is a chemical compound with the formula 32NO . This heterocycle is a red-orange, sublimable solid. As a stable radical, it has applications throughout chemistry and biochemistry. TEMPO was discovered by Lebedev and Kazarnowskii in 1960...
.

Potassium permanganate
Potassium permanganate (KMnO4) is a very strong oxidant able to react with many functional groups, such as secondary alcohols, 1,2-diols, aldehydes, alkenes, oximes, sulfides and thiols. Under controlled conditions, KMnO4 oxidizes very efficiently primary alcohols to carboxylic acids. This reaction, which was first described in detail by Fournier, is typically carried out by adding KMnO4 to a solution or suspension of the alcohol in an alkaline aqueous solution. The resulting mixture is stirred till the oxidation is complete. For the reaction to proceed efficiently, the alcohol must be at least partially dissolved in the aqueous solution. This can be facilitated by the addition of an organic co-solvent such as dioxane, pyridinePyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
, acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
or t-BuOH. KMnO4 will readily react with a carbon - carbon double bond before oxidizing a primary alcohol.
Normally, these oxidations are performed under strong alkaline conditions using a ca. 1N NaOH or KOH
Potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
solution, because this promotes a greater oxidation speed and selectivity. In substrates sensitive to strong base, the reaction can be carried out at a lower pH – or even under acidic conditions — at the cost of a greatly decreased reaction velocity.

Jones oxidation
The so-called Jones reagent is prepared by dissolving chromium trioxideChromium trioxide
Chromium trioxide is the inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name.This compound is a dark-red/orange brown solid, which dissolves in water concomitant with hydrolysis...
(CrO3) in aqueous sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
, which results in formation of a reddish solution containing chromic acid (H2CrO4) and oligomers thereof. Addition of Jones reagent to a solution of a primary alcohol in acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
— as first described by Jones —
results in oxidation of the alcohol to a carboxylic acid. This classical protocol, involving a direct addition, is used very often regardless of the fact that it frequently leads to the formation of substantial amounts of ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s — possessing the structure R-CO-O-CH2-R — derived from oxidative dimerization of primary alcohols. Holland and Gilman proved that this side reaction can be greatly suppressed by following the inverse addition protocol whereby a solution of the primary alcohol in acetone is slowly added to Jones reagent under conditions as dilute as practical.
Jones reagent interacts with secondary alcohols resulting in oxidation to ketones. Treatment of compounds, containing both primary and secondary alcohols, with Jones reagent leads to formation of ketoacids.
Problems encountered with the use of large quantities of chromium trioxide, which is toxic and dangerous for the environment, prompted the development by Zhao of a catalytic procedure, involving treatment with excess of periodic acid
Periodic acid
Periodic acid, or iodic acid is an oxoacid of iodine having chemical formula HIO4 or H5IO6.In dilute aqueous solution, periodic acid exists as discrete hydronium and metaperiodate ions. When more concentrated, orthoperiodic acid, H5IO6, is formed; this dissociates into hydronium and...
(H5IO6) in presence of about 1.2 mol% of CrO3. Zhao's procedure for the use of catalytic CrO3 is very well-suited for reactions on a large scale.

PDC in DMF (Corey and Schmidt)
Pyridinium dichromate (PDC) is a bright-orange solid with the formulae (C5H5NH)2Cr2O7 that is very often used for the oxidation of primary and secondary alcohols to aldehydes and ketones respectively. On the other hand, in 1979, Corey and Schmidt reported that reaction of saturated primary alcohols with PDC, using dimethylformamide (Me2NCHO, DMF) as solvent, results in oxidation to carboxylic acids rather than aldehydes. Interestingly, no oxidation to carboxylic acids occurs on allylic and benzylic primary alcohols. The procedure of Corey and Schmidt for the oxidation of saturated primary alcohols to carboxylic acids is run under essentially neutral conditions.Heyns oxidation
In the heyns oxidation the oxidizing reagent is a combination of oxygen and platinumPlatinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
.
Two-step oxidation of alcohols to acids via isolated aldehydes
As a lot of the aforementioned conditions for the oxidations of primary alcohols to acids are harsh and not compatible with common protection groups, organic chemists often use a two-step procedure for the oxidation to acids.The primary alcohol is oxidized to an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
using one of the many existing procedures (e.g. IBX oxidation, Dess-Martin-Periodinane). The aldehyde can then be subjected to the conditions of the Pinnick oxidation
Pinnick oxidation
The Pinnick oxidation is also known as Lindgren oxidation. It is an organic reaction by which aldehydes can be oxidized into its corresponding carboxylic acid, originally developed by Lindgren and Nilsson. The typical reaction condition used today was modified by G. A. Kraus even before Pinnick. ...
using sodium chlorite
Sodium chlorite
Sodium chlorite is a chemical compound used in the manufacture of paper.-Use:The main application of sodium chlorite is the generation of chlorine dioxide for bleaching and stripping of textiles, pulp, and paper. It is also used for disinfection of a few municipal water treatment plants after...
.
This sequence is often used in natural product synthesis, Nicolaou et al. applied it in their synthesis of Platencin.

