
Oxalyl-CoA decarboxylase
Encyclopedia
In enzymology, an oxalyl-CoA decarboxylase (OXC) is an enzyme
primarily produced by the gastrointestinal bacterium
Oxalobacter formigenes
that catalyzes
the chemical reaction
OXC belongs to the family of lyase
s, specifically the carboxy-lyases, which cleave carbon-carbon bonds. The systematic name of this enzyme class is oxalyl-CoA carboxy-lyase (formyl-CoA-forming). Other names in common use include oxalyl coenzyme A decarboxylase, and oxalyl-CoA carboxy-lyase. This enzyme participates in glyoxylate and dicarboxylate metabolism
. It employs one cofactor
, thiamin diphosphate (TPP), and plays a key role in catabolism of oxalate
, a highly toxic compound that is a product of the oxidation of carbohydrates in many bacteria and plants. Oxalyl-CoA decarboxylase is extremely important for the elimination of ingested oxalates found in human foodstuffs like coffee
, tea
, and chocolate
, and the ingestion of such foods in the absence of Oxalobacter formigenes in the gut can result in kidney disease or even death as a result of oxalate poisoning.
, a TPP-dependent enzyme responsible for the biosynthesis of branched chain amino acids in certain organisms. Sequence alignments between the two enzymes support this claim, as do the presence of vestigial FAD
-binding pockets that play no role in either enzyme’s catalytic activity. The binding of FAD at this site in acetolactate synthase and the binding of ADP
at a cognate site in OXC are thought to play roles in the stabilization of the tertiary structures of the proteins. No FAD binding is observed in oxalyl-CoA decarboxylase, but an excess of coenzyme A
in the crystal structure has led to the hypothesis that the binding site was co-opted during OXC evolution to bind the CoA moiety of its substrate. Despite their similarities, only oxalyl-CoA decarboxylase is necessary for the formation of ATP in Oxalobacter formigenes, and exogenous ADP has been been demonstrated to increase the decarboxylase activity of OXC, but not acetolactate synthase.
ring, which has a pKa near 10. This carbon center ionizes to form a carbanion
, which adds to the carbonyl
group of oxalyl-CoA. This addition is followed by the decarboxylation
of oxalyl-CoA, and then the oxidation and removal of formyl-CoA to regenerate the carbanion form of TPP. While the reaction mechanism is shared with other TPP-dependent enzymes, the residues found in the active site of OXC are unique, which has raised questions about whether TDP must be deprotonated by a basic amino acid at a second site away from the carbanion-forming site to activate the cofactor.
As of late 2007, 6 structures
have been solved for this class of enzymes, with PDB
accession codes , , , , , and .
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
primarily produced by the gastrointestinal bacterium
Gut flora
Gut flora consists of microorganisms that live in the digestive tracts of animals and is the largest reservoir of human flora. In this context, gut is synonymous with intestinal, and flora with microbiota and microflora....
Oxalobacter formigenes
Oxalobacter formigenes
Oxalobacter formigenes is an oxalate-degrading anaerobic bacterium that colonizes the large intestine of numerous vertebrates, including humans. O. formigenes and humans share a beneficial symbiosis....
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- oxalyl-CoA
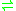 formyl-CoA + CO2
formyl-CoA + CO2
OXC belongs to the family of lyase
Lyase
In biochemistry, a lyase is an enzyme that catalyzes the breaking of various chemical bonds by means other than hydrolysis and oxidation, often forming a new double bond or a new ring structure...
s, specifically the carboxy-lyases, which cleave carbon-carbon bonds. The systematic name of this enzyme class is oxalyl-CoA carboxy-lyase (formyl-CoA-forming). Other names in common use include oxalyl coenzyme A decarboxylase, and oxalyl-CoA carboxy-lyase. This enzyme participates in glyoxylate and dicarboxylate metabolism
Glyoxylate and dicarboxylate metabolism
Glyoxylate and dicarboxylate metabolism describes a variety of reactions involving glyoxylate or dicarboxylates. Glyoxylate is the conjugate base of glyoxylic acid, and within a buffered environment of known pH such as the cell cytoplasm these terms can be used almost interchangeably, as the gain...
. It employs one cofactor
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
, thiamin diphosphate (TPP), and plays a key role in catabolism of oxalate
Oxalate
Oxalate , is the dianion with formula C2O42− also written 22−. Either name is often used for derivatives, such as disodium oxalate, 2C2O42−, or an ester of oxalic acid Oxalate (IUPAC: ethanedioate), is the dianion with formula C2O42− also written (COO)22−. Either...
, a highly toxic compound that is a product of the oxidation of carbohydrates in many bacteria and plants. Oxalyl-CoA decarboxylase is extremely important for the elimination of ingested oxalates found in human foodstuffs like coffee
Coffee
Coffee is a brewed beverage with a dark,init brooo acidic flavor prepared from the roasted seeds of the coffee plant, colloquially called coffee beans. The beans are found in coffee cherries, which grow on trees cultivated in over 70 countries, primarily in equatorial Latin America, Southeast Asia,...
, tea
Tea
Tea is an aromatic beverage prepared by adding cured leaves of the Camellia sinensis plant to hot water. The term also refers to the plant itself. After water, tea is the most widely consumed beverage in the world...
, and chocolate
Chocolate
Chocolate is a raw or processed food produced from the seed of the tropical Theobroma cacao tree. Cacao has been cultivated for at least three millennia in Mexico, Central and South America. Its earliest documented use is around 1100 BC...
, and the ingestion of such foods in the absence of Oxalobacter formigenes in the gut can result in kidney disease or even death as a result of oxalate poisoning.
Evolution
Oxalyl-CoA decarboxylase is hypothesized to be evolutionarily related to acetolactate synthaseAcetolactate synthase
The acetolactate synthase enzyme is a protein found in plants and micro-organisms. ALS catalyzes the first step in the synthesis of the branched-chain amino acids ....
, a TPP-dependent enzyme responsible for the biosynthesis of branched chain amino acids in certain organisms. Sequence alignments between the two enzymes support this claim, as do the presence of vestigial FAD
FAD
In biochemistry, flavin adenine dinucleotide is a redox cofactor involved in several important reactions in metabolism. FAD can exist in two different redox states, which it converts between by accepting or donating electrons. The molecule consists of a riboflavin moiety bound to the phosphate...
-binding pockets that play no role in either enzyme’s catalytic activity. The binding of FAD at this site in acetolactate synthase and the binding of ADP
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
at a cognate site in OXC are thought to play roles in the stabilization of the tertiary structures of the proteins. No FAD binding is observed in oxalyl-CoA decarboxylase, but an excess of coenzyme A
Coenzyme A
Coenzyme A is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All sequenced genomes encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it as a substrate...
in the crystal structure has led to the hypothesis that the binding site was co-opted during OXC evolution to bind the CoA moiety of its substrate. Despite their similarities, only oxalyl-CoA decarboxylase is necessary for the formation of ATP in Oxalobacter formigenes, and exogenous ADP has been been demonstrated to increase the decarboxylase activity of OXC, but not acetolactate synthase.
Reaction Mechanism
A key feature of the cofactor TPP is the relatively acidic proton bound to the carbon atom between the nitrogen and sulfur in the thiazoleThiazole
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen; the term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS...
ring, which has a pKa near 10. This carbon center ionizes to form a carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
, which adds to the carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group of oxalyl-CoA. This addition is followed by the decarboxylation
Decarboxylation
Decarboxylation is a chemical reaction that releases carbon dioxide . Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carbonation, the addition of CO2 to...
of oxalyl-CoA, and then the oxidation and removal of formyl-CoA to regenerate the carbanion form of TPP. While the reaction mechanism is shared with other TPP-dependent enzymes, the residues found in the active site of OXC are unique, which has raised questions about whether TDP must be deprotonated by a basic amino acid at a second site away from the carbanion-forming site to activate the cofactor.
Structure
Oxalyl-CoA decarboxylase is tetrameric, and each monomer consists of three α/β-type domains. The thiamine diphosphate-binding site rests on the subunit-subunit interface between two of the domains, which is commonly seen in its class of enzymes. Oxalyl-CoA decarboxylase is structurally homologous to acetolactate synthase found in plants and other microorganisms, but OXC binds ADP in a region that is similar to the FAD-binding site in acetolactate synthase.As of late 2007, 6 structures
Tertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
have been solved for this class of enzymes, with PDB
Protein Data Bank
The Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids....
accession codes , , , , , and .

