Organochromium chemistry
Encyclopedia
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
that deals with organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s containing a chromium
Chromium
Chromium is a chemical element which has the symbol Cr and atomic number 24. It is the first element in Group 6. It is a steely-gray, lustrous, hard metal that takes a high polish and has a high melting point. It is also odorless, tasteless, and malleable...
to carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
bond and their reactions. The field is of some relevance to organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. The relevant oxidation states for chromium range from -2 to +6.
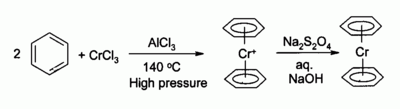
History
The first organochromium compound was described in 1919 by Franz HeinFranz Hein
Franz Hein was a German scientist. One of his notable contributions is in the discovery of π- complexes of chromium.-History:...
. He treated phenylmagnesium bromide
Phenylmagnesium bromide
Phenylmagnesium bromide, with the simplified formula , is a magnesium-containing organometallic compound. It is so commonly used that it is commercially available as a solution in diethyl ether or tetrahydrofuran . Phenylmagnesium bromide is a Grignard reagent...
with chromium(III) chloride
Chromium(III) chloride
Chromium chloride is a violet coloured solid with the formula CrCl3. The most common form of CrCl3 sold commercially is a dark green hexahydrate with the formula [CrCl24]Cl.2H2O. Two other hydrates are known, pale green [CrCl5]Cl2.H2O and violet [Cr6]Cl3...
to give a new product (after hydrolysis) which he incorrectly identified as pentaphenyl chromium bromide (Ph5CrBr). Years later, in 1957 H.H. Zeiss et al. repeated Hein's experiments and correctly arrived at a cationic bisarene chromium sandwich compound
Sandwich compound
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic covalent bonds to two arene ligands. The arenes have the formula CnHn, substituted derivatives and heterocyclic derivatives...
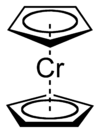
(Ar2Cr+). Bis(benzene)chromium
Bis(benzene)chromium
Bischromium is the organometallic compound with the formula Cr2. It is sometimes called dibenzenechromium. The compound played an important role in the development of sandwich compounds in organometallic chemistry and is the prototypical complex containg two arene ligands.-Preparation:The...
itself was discovered around the same time in 1956 by Ernst Otto Fischer
Ernst Otto Fischer
Ernst Otto Fischer was a German chemist who won the Nobel Prize for pioneering work in the area of organometallic chemistry.-Early life:...
by reaction of chromium(III) chloride
Chromium(III) chloride
Chromium chloride is a violet coloured solid with the formula CrCl3. The most common form of CrCl3 sold commercially is a dark green hexahydrate with the formula [CrCl24]Cl.2H2O. Two other hydrates are known, pale green [CrCl5]Cl2.H2O and violet [Cr6]Cl3...
, benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
and aluminum chloride. The related compound chromocene
Chromocene
Chromocene is an organochromium compound with the formula Cr2, often abbreviated Cp2Cr. This sandwich compound is structurally similar to ferrocene but does not follow the 18-electron rule because it only has 16 electrons. It also paramagnetic and highly reducing...
was discovered a few years earlier in 1953 also by Fischer .
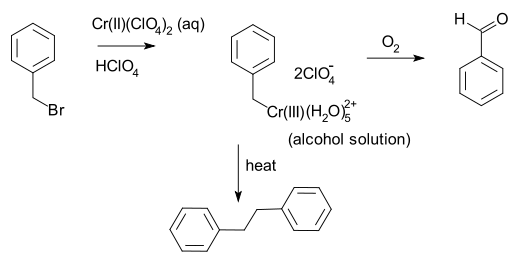
Benzyl bromide
Benzyl bromide, or α-bromotoluene, is an organic compound consisting of a benzene ring substituted with a bromomethyl group. It can be prepared by the bromination of toluene at room temperature in air, using manganese oxide as a heterogeneous catalyst...
and chromium
Chromium
Chromium is a chemical element which has the symbol Cr and atomic number 24. It is the first element in Group 6. It is a steely-gray, lustrous, hard metal that takes a high polish and has a high melting point. It is also odorless, tasteless, and malleable...
(II) perchlorate
Perchlorate
Perchlorates are the salts derived from perchloric acid . They occur both naturally and through manufacturing. They have been used as a medicine for more than 50 years to treat thyroid gland disorders. They are used extensively within the pyrotechnics industry, and ammonium perchlorate is also a...
. This reaction involves one-electron oxidative addition
Oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre...
of the carbon-bromine bond, a process which was shown by Kochi to be a case of double single electron transfer, first to give the benzyl
Benzyl
In organic chemistry, benzyl is the term used to describe the substituent or molecular fragment possessing the structure C6H5CH2-. Benzyl features a benzene ring attached to a CH2 group.-Nomenclature:...
free radical and then to the benzyl anion.
G. Wilke et al. introduced tris-(η-allyl)chromium in 1963 as an early Ziegler-Natta catalyst
Ziegler-Natta catalyst
A Ziegler–Natta catalyst is a catalyst used in the synthesis of polymers of 1-alkenes . Three types of Ziegler–Natta catalysts are currently employed:* Solid and supported catalysts based on titanium compounds...
(but not successful in the long run) Chromocene compounds were first employed in ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
in 1972 by Union Carbide
Union Carbide
Union Carbide Corporation is a wholly owned subsidiary of The Dow Chemical Company. It currently employs more than 2,400 people. Union Carbide primarily produces chemicals and polymers that undergo one or more further conversions by customers before reaching consumers. Some are high-volume...
and continue to used today in the industrial production of high-density polyethylene.
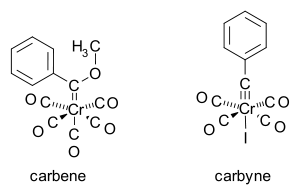
The organochromium compound (phenylmethoxycarbene)pentacarbonylchromium, Ph(OCH3)C=Cr(CO)5 was the first carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
complex to be crystallographically characterized by Fischer in 1967 (now called a Fischer carbene). The first ever carbyne
Carbyne
In chemistry, a carbyne is a monovalent carbon radical species containing an electrically neutral univalent carbon atom with three non-bonded electrons.- Gas phase/reactive intermediate :...
, this one also containing chromium, made its debut in 1973.
The first example of a proposed metal-metal quintuple bond
Quintuple bond
A quintuple bond in chemistry is an unusual type of chemical bond first reported in 2005 for a dichromium compound. Single bonds, double bonds, and triple bonds are commonplace in chemistry. Quadruple bonds are rarer but occur especially for Cr, Mo, W, and Re, e.g. [Mo2Cl8]4− and [Re2Cl8]2−...
is found in a compound of the type [CrAr]2, where Ar is a bulky aryl ligand.
Applications in organic synthesis
Although organochromium chemistry is heavily employed in industrial catalysis, relatively few reagents have been developed for applications in organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. Two are the Nozaki-Hiyama-Kishi reaction (1977) (transmetallation with organonickel intermediate) and the Takai olefination
Takai olefination
Takai olefination in organic chemistry describes the organic reaction of an aldehyde with a diorganochromium compound to form an alkene. In the original 1986 publication the aldehyde is benzaldehyde and the organochromium species is generated from iodoform or bromoform and an excess of chromium...
(1986)(oxidation of Cr(II) to Cr(III) while replacing halogens).
Organochromium compounds
Organochromium compounds can be divided into these broad compound classes:- Sandwich compoundSandwich compoundIn organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic covalent bonds to two arene ligands. The arenes have the formula CnHn, substituted derivatives and heterocyclic derivatives...
s: chromoceneChromoceneChromocene is an organochromium compound with the formula Cr2, often abbreviated Cp2Cr. This sandwich compound is structurally similar to ferrocene but does not follow the 18-electron rule because it only has 16 electrons. It also paramagnetic and highly reducing...
s (Cp2)Cr and Bis(benzene)chromiumBis(benzene)chromiumBischromium is the organometallic compound with the formula Cr2. It is sometimes called dibenzenechromium. The compound played an important role in the development of sandwich compounds in organometallic chemistry and is the prototypical complex containg two arene ligands.-Preparation:The...
derivatives (ArH2)Cr. More commonly studied are the half-sandwich complexes (ArH)Cr(CO)3. - Chromium carbeneCarbeneIn chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
s (R1)R2)C::CrLn and carbyneCarbyneIn chemistry, a carbyne is a monovalent carbon radical species containing an electrically neutral univalent carbon atom with three non-bonded electrons.- Gas phase/reactive intermediate :...
s (R:::CrLn) - Chromium(III) complexes RCrL5.
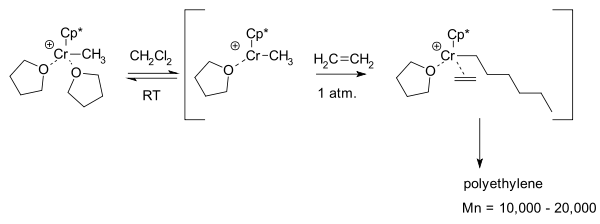
Ethylene polymerization and oligomerization
Chromonium catalysts are important in ethyleneEthylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
. Two industrial catalysts exist: the Phillips catalyst is deposited chromium(III) oxide
Chromium(III) oxide
Chromium oxide is the inorganic compound of the formula Cr2O3. It is one of principal oxides of chromium and is used as a pigment. In nature, it occurs as the rare mineral eskolaite.-Structure and properties:...
on silica with activation by hydrogen. A Union Carbide
Union Carbide
Union Carbide Corporation is a wholly owned subsidiary of The Dow Chemical Company. It currently employs more than 2,400 people. Union Carbide primarily produces chemicals and polymers that undergo one or more further conversions by customers before reaching consumers. Some are high-volume...
catalyst is based on silica and chromocene
Chromocene
Chromocene is an organochromium compound with the formula Cr2, often abbreviated Cp2Cr. This sandwich compound is structurally similar to ferrocene but does not follow the 18-electron rule because it only has 16 electrons. It also paramagnetic and highly reducing...
. Exactly how these catalysts work is unclear. One model system describes it as coordination polymerization
Coordination polymerization
Coordination polymerization is a form of addition polymerization in which monomer adds to a growing macromolecule through an organometallic active center...
:
With two THF
ThF
Follicular B helper T cells , are antigen-experienced CD4+ T cells found in the B cell follicles of secondary lymphoid organs such as lymph nodes, spleens and Peyer's patches, and are identified by their constitutive expression of the B cell follicle homing receptor CXCR5...
ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s the catalyst is stable but in dichloromethane
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
one ligand is lost to form a 13 electron chromium intermediate. This enables side-on addition of a ethylene unit and a polymer chain can grow by migratory insertion
Migratory insertion
A migratory insertion is a type of reaction in organometallic chemistry wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiated by the mechanism that leads to the resulting stereochemistry of the...
.
Chromium compounds also catalyse the trimerization of ethylene to produce the monomer 1-hexene
1-Hexene
1-Hexene is an organic compound with the formula CH2CHC4H9. It is an alkene that is classified in industry as higher olefin and an alpha-olefin, the latter term meaning that the double bond is located at the alpha position, endowing the compound with higher reactivity and thus useful chemical...
.
Comparisons with heavier group 6 organometallics
The heavier group 6 elements molybdenumMolybdenum
Molybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores...
and tungsten
Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
form organometallic compounds similar to those for chromium but also with differences. Whereas Cr(III) aquo alkyl compounds are well studied, the corresponding Mo(III) and W(III) compounds are not. Whereas chromocene is a stable compound, the related molybdenocene and tungstenocene are highly reactive. On the other hand, Mo and W readily form derivatives of the type Cp2MX2, whereas the smaller Cr does not form such clamshell compounds. Homoleptic alkyl and aryl complexes of the type R4M are rare, and hexamethyltungsten has no analogue in Cr chemistry.
Similar are the carbonyls such as molybdenum hexacarbonyl
Molybdenum hexacarbonyl
Molybdenum hexacarbonyl is the chemical compound with the formula Mo6. This colorless solid, like its chromium and tungsten analogues, is noteworthy as a volatile, air-stable derivative of a metal in its zero oxidation state.-Structure and properties:Mo6 adopts an octahedral geometry consisting...
and tungsten hexacarbonyl
Tungsten hexacarbonyl
Tungsten hexacarbonyl is the chemical compound with the formula W6. This complex gave rise to the first example of a dihydrogen complex....
and the related carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
and carbyne
Carbyne
In chemistry, a carbyne is a monovalent carbon radical species containing an electrically neutral univalent carbon atom with three non-bonded electrons.- Gas phase/reactive intermediate :...
complexes. Compounds of the type [CpM(CO)3]2 are known for all three metals, e.g. Cyclopentadienylmolybdenum tricarbonyl dimer
Cyclopentadienylmolybdenum tricarbonyl dimer
Cyclopentadienylmolybdenum tricarbonyl dimer is the chemical compound with the formula Cp2Mo26, where Cp is C5H5. This a dark red crystalline solid is air stable in solid form, but decomposes in solution when exposed to air...
. The chromium compound is however prone to homolysis of the Cr-Cr bond owing to steric crowding.
In the Kauffmann olefination
Kauffmann olefination
The Kauffmann olefination is a chemical reaction to convert aldehydes and ketones to methylene-olefins. This reaction was discovered by the German chemist Thomas Kauffmann and is related to the better known Tebbe olefination or Wittig reaction....
, molybdenum(III) chloride and methyllithium form an organometallic complex capable of carbonyl olefination.
See also
- Other bonds of carbon with elements in the periodic table: