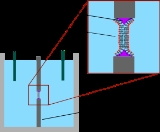
Model lipid bilayer
Encyclopedia
A model lipid bilayer is any bilayer assembled in vitro
, as opposed to the bilayer of natural cell membrane
s or covering various sub-cellular structures like the nucleus
. A model bilayer can be made with either synthetic or natural lipids. The simplest model systems contain only a single pure synthetic lipid. More physiologically relevant model bilayers can be made with mixtures of several synthetic or natural lipids.
There are many different types of model bilayers, each having experimental advantages and disadvantages. The first system developed was the black lipid membrane or “painted” bilayer, which allows simple electrical characterization of bilayers but is short-lived and can be difficult to work with. Supported bilayers are anchored to a solid substrate, increasing stability and allowing the use of characterization tools not possible in bulk solution. These advantages come at the cost of unwanted substrate interactions which can denature membrane proteins.
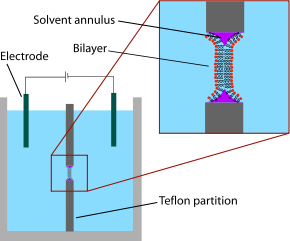 The earliest model bilayer system developed was the “painted” bilayer, also known as a “black lipid membrane.” The term “painted” refers to the process by which these bilayers are made. First, a small aperture is created in a hydrophobic material such as Teflon. Typically the diameter of this hole is a few tens of micrometers. A solution of lipids dissolved in an organic solvent is then applied with a brush or a syringe across the aperture. The solvent used must have a very low partition coefficient
The earliest model bilayer system developed was the “painted” bilayer, also known as a “black lipid membrane.” The term “painted” refers to the process by which these bilayers are made. First, a small aperture is created in a hydrophobic material such as Teflon. Typically the diameter of this hole is a few tens of micrometers. A solution of lipids dissolved in an organic solvent is then applied with a brush or a syringe across the aperture. The solvent used must have a very low partition coefficient
in water and must be relatively viscous to prevent immediate rupture. The most common solvent used is a mixture of decane
and squalene
. A lipid monolayer spontaneously forms at the interface between the organic and aqueous phases on either side of the lipid/solvent droplet. Because the walls of the aperture are hydrophobic the lipid/solvent solution wets this interface, thinning the droplet in the center. Once the two sides of the droplet come close enough together, the lipid monolayers fuse, rapidly excluding the small remaining volume of solution. At this point a bilayer is formed in the center of the aperture, but a significant annulus of solvent remains at the perimeter. This annulus is required to maintain stability by acting as a bridge between the ~5 nm bilayer and the 10's of micrometer thick sheet in which the aperture is made.
The term “black” bilayer refers to the fact that they are dark in reflected light because the thickness of the membrane is only a few nanometers, so light reflecting off the back face destructively interferes with light reflecting off the front face. Indeed, this was one of the first clues that this technique produced a membrane of molecular-scale thickness. Black lipid membranes are also well suited to electrical characterization because the two chambers separated by the bilayer are both accessible, allowing simple placement of large electrodes. For this reason, electrical characterization is one of the most important methods used in conjunction with painted lipid bilayers. Simple measurements indicate when a bilayer forms and when it breaks, as an intact bilayer has a large resistance (>GΩ) and a large capacitance (~2µF/cm2). More advanced electrical characterization has been particularly important in the study of voltage gated ion channels
. Membrane proteins such as ion channels typically cannot be incorporated directly into the painted bilayer during formation because immersion in an organic solvent would denature the protein. Instead, the protein is solubilized with a detergent
and added to the aqueous solution after the bilayer is formed. The detergent coating allows these proteins to spontaneously insert into the bilayer over a period of minutes. First experiments have been performed to combine electrophysiological and structural investigations of Black Lipid Membranes.
The main problems associated with painted bilayers are residual solvent and limited lifetime. Some researchers believe that pockets of solvent trapped between the two bilayer leaflets can disrupt normal protein function. To overcome this limitation, Montal and Mueller developed a modified deposition technique that eliminates the use of a heavy non-volatile solvent. In this method, the aperture starts out above the water surface, completely separating the two fluid chambers. On the surface of each chamber, a monolayer is formed by applying lipids in a volatile solvent such as chloroform
and waiting for the solvent to evaporate. The aperture is then lowered through the air-water interface and the two monolayers from the separate chambers are folded down against each other, forming a bilayer across the aperture. The stability issue has proven more difficult to solve. Typically, a black lipid membrane will survive for less than an hour, precluding long-term experiment
s. This lifetime can be extended by precisely structuring the supporting aperture,chemically crosslinking the lipids or gelling the surrounding solution to mechanically support the bilayer. Work is ongoing in this area and lifetimes of several hours will become feasible.
 Unlike a vesicle or a cell membrane in which the lipid bilayer is rolled into an enclosed shell, a supported bilayer is a planar structure sitting on a solid support. Because of this, only the upper face of the bilayer is exposed to free solution. This layout has advantages and drawbacks related to the study of lipid bilayers. One of the greatest advantages of the supported bilayer is its stability. SLBs will remain largely intact even when subject to high flow rates or vibration and, unlike black lipid membranes, the presence of holes will not destroy the entire bilayer. Because of this stability, experiments lasting weeks and even months are possible with supported bilayers while BLM experiments are usually limited to hours. Another advantage of the supported bilayer is that, because it is on a flat hard surface, it is amenable to a number of characterization tools which would be impossible or would offer lower resolution if performed on a freely floating sample.
Unlike a vesicle or a cell membrane in which the lipid bilayer is rolled into an enclosed shell, a supported bilayer is a planar structure sitting on a solid support. Because of this, only the upper face of the bilayer is exposed to free solution. This layout has advantages and drawbacks related to the study of lipid bilayers. One of the greatest advantages of the supported bilayer is its stability. SLBs will remain largely intact even when subject to high flow rates or vibration and, unlike black lipid membranes, the presence of holes will not destroy the entire bilayer. Because of this stability, experiments lasting weeks and even months are possible with supported bilayers while BLM experiments are usually limited to hours. Another advantage of the supported bilayer is that, because it is on a flat hard surface, it is amenable to a number of characterization tools which would be impossible or would offer lower resolution if performed on a freely floating sample.
One of the clearest examples of this advantage is the use of mechanical probing techniques which require a direct physical interaction with the sample. Atomic force microscopy (AFM) has been used to image lipid phase separation
, formation of transmembrane nanopores followed by single protein molecule adsorption, and protein assembly with sub-nm accuracy without the need for a labeling dye. More recently, AFM has also been used to directly probe the mechanical properties
of single bilayers and to perform force spectroscopy on individual membrane proteins. These studies would be difficult or impossible without the use of supported bilayers since the surface of a cell or vesicle is relatively soft and would drift and fluctuate over time. Another example of a physical probe is the use of the quartz crystal microbalance
(QCM) to study binding kinetics at the bilayer surface. Dual polarisation interferometry
is a high resolution optical tool for characterising the order and disruption in lipid bilayers during interactions or phase transitions providing complementary data to QCM measurements .
Many modern fluorescence microscopy techniques also require a rigidly-supported planar surface. Evanescent field
methods such as total internal reflection fluorescence microscopy (TIRF) and surface plasmon resonance
(SPR) can offer extremely sensitive measurement of analyte binding and bilayer optical properties but can only function when the sample is supported on specialized optically functional materials. Another class of methods applicable only to supported bilayers is those based on optical interference such as fluorescence interference contrast microscopy (FLIC) and reflection interference contrast microscopy (RICM). When the bilayer is supported on top of a reflective surface, variations in intensity due to destructive interference from this interface can be used to calculate with angstrom accuracy the position of fluorophores within the bilayer. Both evanescent and interference techniques offer sub-wavelength resolution in only one dimension (z, or vertical). In many cases, this resolution is all that is needed. After all, bilayers are very small only in one dimension. Laterally, a bilayer can extend for many micrometres or even millimeters. But certain phenomena like dynamic phase rearrangement do occur in bilayers on a lateral sub-micrometre lengthscale. A promising approach to studying these structures is near field scanning optical microscopy (NSOM). Like AFM, NSOM relies on the scanning of a micromachined tip to give a highly localized signal. But unlike AFM, NSOM uses an optical rather than physical interaction with the sample, potentially perturbing delicate structures to a lesser extent.
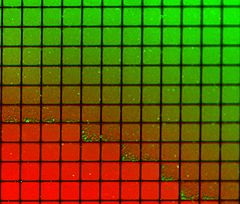 Another important capability of supported bilayers is the ability to pattern the surface to produce multiple isolated regions on the same substrate. This phenomenon was first demonstrated using scratches or metallic “corrals” to prevent mixing between adjacent regions while still allowing free diffusion within any one region. Later work extended this concept by integrating microfluidics
Another important capability of supported bilayers is the ability to pattern the surface to produce multiple isolated regions on the same substrate. This phenomenon was first demonstrated using scratches or metallic “corrals” to prevent mixing between adjacent regions while still allowing free diffusion within any one region. Later work extended this concept by integrating microfluidics
to demonstrate that stable composition gradients could be formed in bilayers, potentially allowing massively parallel studies of phase segregation, molecular binding and cellular response to artificial lipid membranes. Creative utilization of the corral concept has also allowed studies of the dynamic reorganization of membrane proteins at the synaptic
interface.
One of the primary limitations of supported bilayers is the possibility of unwanted interactions with the substrate. Although supported bilayers generally do not directly touch the substrate surface, they are separated by only a very thin water gap. The size and nature of this gap depends on the substrate material and lipid species but is generally about 1 nm for zwitterionic lipids supported on silica, the most common experimental system. Because this layer is so thin there is extensive hydrodynamic coupling between the bilayer and the substrate, resulting in a lower diffusion coefficient in supported bilayers than for free bilayers of the same composition. A certain percentage of the supported bilayer will also be completely immobile, although the exact nature of and reason for these “pinned” sites is still uncertain. For high quality liquid phase supported bilayers the immobile fraction is typically around 1-5%. To quantify the diffusion coefficient and mobile fraction, researchers studying supported bilayers will often report FRAP
data.
Unwanted substrate interactions are a much greater problem when incorporating integral membrane proteins, particularly those with large domains sticking out beyond the core of the bilayer. Because the gap between bilayer and substrate is so thin these proteins will often become denatured
on the substrate surface and therefore lose all functionality. One approach to circumvent this problem is the use of polymer tethered bilayers. In these systems the bilayer is supported on a loose network of hydrated polymers or hydrogel which acts as a spacer and theoretically prevents denaturing substrate interactions. In practice, some percentage of the proteins will still lose mobility and functionality, probably due to interactions with the polymer/lipid anchors. Research in this area is ongoing.
Gold can be used as a substrate because of its inert chemistry and thiolipids for covalent binding to the gold. Thiolipids are composed of lipid derivatives, extended at their polar head-groups by hydrophilic spacers which terminate in a thiol or disulphide group that forms a covalent bond with gold, forming self assembled monolayers (SAM).
The limitation of the intra-membrane mobility of supported lipid bilayers can be overcome by introducing half-membrane spanning tether lipids with benzyl disulphide (DPL) and synthetic archaea analogue full membrane spanning lipids with phytanoly chains to stabilize the structure and polyethyleneglycol units as a hydrophilic spacer. Bilayer formation is achieved either by exposure of the lipid coated gold substrate to outer layer lipids either in an ethanol solution or in liposomes
. The advantage of this approach is that the because of the hydrophilic space of around 4 nm, the interaction with the substrate is minimal and the extra space allows the introduction of protein ion channels into the bilayer. Additionally the spacer layer creates an ionic reservoir . that readily enables ac electrical impedance measurement across the bilayer.
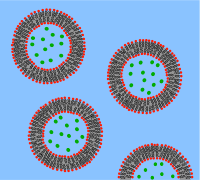 A vesicle is a lipid bilayer rolled up into a spherical shell, enclosing a small amount of water and separating it from the water outside the vesicle. Because of this fundamental similarity to the cell membrane, vesicles have been used extensively to study the properties of lipid bilayers. Another reason vesicles have been used so frequently is that they are relatively easy to make. If a sample of dehydrated lipid is exposed to water it will spontaneously form vesicles. These initial vesicles are typically multilamellar (many-walled) and are of a wide range of sizes from tens of nanometers to several micrometres. Methods such as sonication or extrusion through a membrane are needed to break these initial vesicles into smaller, single-walled vesicles of uniform diameter known as small unilamellar vesicles (SUVs). SUVs are typically between 50 and 200 nm diameter. Alternatively, rather than synthesizing vesicles it is possible to simply isolate them from cell cultures or tissue samples. Vesicles are used to transport lipids, proteins and many other molecules within the cell as well as into or out of the cell. These naturally isolated vesicles are composed of a complex mixture of different lipids and proteins so, although they offer greater realism for studying specific biological phenomena, simple artificial vesicles are preferred for studies of fundamental lipid properties.
A vesicle is a lipid bilayer rolled up into a spherical shell, enclosing a small amount of water and separating it from the water outside the vesicle. Because of this fundamental similarity to the cell membrane, vesicles have been used extensively to study the properties of lipid bilayers. Another reason vesicles have been used so frequently is that they are relatively easy to make. If a sample of dehydrated lipid is exposed to water it will spontaneously form vesicles. These initial vesicles are typically multilamellar (many-walled) and are of a wide range of sizes from tens of nanometers to several micrometres. Methods such as sonication or extrusion through a membrane are needed to break these initial vesicles into smaller, single-walled vesicles of uniform diameter known as small unilamellar vesicles (SUVs). SUVs are typically between 50 and 200 nm diameter. Alternatively, rather than synthesizing vesicles it is possible to simply isolate them from cell cultures or tissue samples. Vesicles are used to transport lipids, proteins and many other molecules within the cell as well as into or out of the cell. These naturally isolated vesicles are composed of a complex mixture of different lipids and proteins so, although they offer greater realism for studying specific biological phenomena, simple artificial vesicles are preferred for studies of fundamental lipid properties.
Since artificial SUVs can be made in large quantities they are suitable for bulk material studies such as x-ray diffraction to determine lattice spacing and differential scanning calorimetry to determine phase transitions. Dual polarisation interferometry
can measure unilamelar and multilamelar structures and insertion into and disruption of the vesciles in a label free assay format . Vesicles can also be labeled with fluorescent dyes to allow sensitive FRET-based fusion
assays. In spite of this fluorescent labeling it is often difficult to perform detailed imaging on SUVs simply because they are so small. To combat this problem researchers have developed the giant unilamellar vesicle (GUV). GUVs are large enough (several tens of micrometres) to study with traditional florescence microscopy. Many of the studies of lipid rafts in artificial lipid systems have been performed with GUVs for this reason. Compared to supported bilayers, GUVs present a more “natural” environment since there is no nearby solid surface to induce defects or denature proteins. However, GUVs are relatively fragile, time consuming to make and can only be produced in limited yield compared to SUVs.
To circumvent these problems a microfluidic assembly line approach to GUVs was reported.
micelles are another class of model membranes that are commonly to purify and study membrane proteins, although they lack a lipid bilayer. In aqueous solutions, micelles are assemblies of amphipathic molecules with their hydrophilic heads exposed to solvent and their hydrophobic tails in the center. Micelles can solubilize membrane proteins by partially encapsulating them and shielding their hydrophobic surfaces from solvent.
Bicelles are a related class of model membrane , typically made of two lipids, one of which forms a lipid bilayer while the other forms an amphipathic, micelle-like assembly shielding the bilayer center from surround solvent molecules. Bicelles can be thought of as a segment of bilayer encapsulated and solubilized by a micelle. Bicelles are much smaller than liposomes, and so can be used in experiments such as NMR
spectroscopy where the larger vesicles are not an option.
Nanodiscs
consist of a segment of bilayer encapsulated by an amphipathic protein coat, rather than a lipid or detergent layer. Nanodiscs are more stable than bicelles and micelles at low concentrations, and are very well-defined in size (depending on the type of protein coat, between 10 and 20 nm). Membrane proteins incorporated into and solubilized by Nanodiscs can be studied by a wide variety of biophysical techniques.
In vitro
In vitro refers to studies in experimental biology that are conducted using components of an organism that have been isolated from their usual biological context in order to permit a more detailed or more convenient analysis than can be done with whole organisms. Colloquially, these experiments...
, as opposed to the bilayer of natural cell membrane
Cell membrane
The cell membrane or plasma membrane is a biological membrane that separates the interior of all cells from the outside environment. The cell membrane is selectively permeable to ions and organic molecules and controls the movement of substances in and out of cells. It basically protects the cell...
s or covering various sub-cellular structures like the nucleus
Cell nucleus
In cell biology, the nucleus is a membrane-enclosed organelle found in eukaryotic cells. It contains most of the cell's genetic material, organized as multiple long linear DNA molecules in complex with a large variety of proteins, such as histones, to form chromosomes. The genes within these...
. A model bilayer can be made with either synthetic or natural lipids. The simplest model systems contain only a single pure synthetic lipid. More physiologically relevant model bilayers can be made with mixtures of several synthetic or natural lipids.
There are many different types of model bilayers, each having experimental advantages and disadvantages. The first system developed was the black lipid membrane or “painted” bilayer, which allows simple electrical characterization of bilayers but is short-lived and can be difficult to work with. Supported bilayers are anchored to a solid substrate, increasing stability and allowing the use of characterization tools not possible in bulk solution. These advantages come at the cost of unwanted substrate interactions which can denature membrane proteins.
Black lipid membranes (BLM)

Partition coefficient
In chemistry and the pharmaceutical sciences, a partition- or distribution coefficient is the ratio of concentrations of a compound in the two phases of a mixture of two immiscible solvents at equilibrium. The terms "gas/liquid partition coefficient" and "air/water partition coefficient" are...
in water and must be relatively viscous to prevent immediate rupture. The most common solvent used is a mixture of decane
Decane
Decane is an alkane hydrocarbon with the chemical formula CH38CH3.75 structural isomers of decane exist, all of which are flammable liquids. Decane is one of the components of gasoline . Like other alkanes, it is nonpolar and therefore will not dissolve in polar liquids such as water...
and squalene
Squalene
Squalene is a natural organic compound originally obtained for commercial purposes primarily from shark liver oil, though plant sources are used as well, including amaranth seed, rice bran, wheat germ, and olives. All plants and animals produce squalene, including humans...
. A lipid monolayer spontaneously forms at the interface between the organic and aqueous phases on either side of the lipid/solvent droplet. Because the walls of the aperture are hydrophobic the lipid/solvent solution wets this interface, thinning the droplet in the center. Once the two sides of the droplet come close enough together, the lipid monolayers fuse, rapidly excluding the small remaining volume of solution. At this point a bilayer is formed in the center of the aperture, but a significant annulus of solvent remains at the perimeter. This annulus is required to maintain stability by acting as a bridge between the ~5 nm bilayer and the 10's of micrometer thick sheet in which the aperture is made.
The term “black” bilayer refers to the fact that they are dark in reflected light because the thickness of the membrane is only a few nanometers, so light reflecting off the back face destructively interferes with light reflecting off the front face. Indeed, this was one of the first clues that this technique produced a membrane of molecular-scale thickness. Black lipid membranes are also well suited to electrical characterization because the two chambers separated by the bilayer are both accessible, allowing simple placement of large electrodes. For this reason, electrical characterization is one of the most important methods used in conjunction with painted lipid bilayers. Simple measurements indicate when a bilayer forms and when it breaks, as an intact bilayer has a large resistance (>GΩ) and a large capacitance (~2µF/cm2). More advanced electrical characterization has been particularly important in the study of voltage gated ion channels
Voltage-gated ion channel
Voltage-gated ion channels are a class of transmembrane ion channels that are activated by changes in electrical potential difference near the channel; these types of ion channels are especially critical in neurons, but are common in many types of cells....
. Membrane proteins such as ion channels typically cannot be incorporated directly into the painted bilayer during formation because immersion in an organic solvent would denature the protein. Instead, the protein is solubilized with a detergent
Detergent
A detergent is a surfactant or a mixture of surfactants with "cleaning properties in dilute solutions." In common usage, "detergent" refers to alkylbenzenesulfonates, a family of compounds that are similar to soap but are less affected by hard water...
and added to the aqueous solution after the bilayer is formed. The detergent coating allows these proteins to spontaneously insert into the bilayer over a period of minutes. First experiments have been performed to combine electrophysiological and structural investigations of Black Lipid Membranes.
The main problems associated with painted bilayers are residual solvent and limited lifetime. Some researchers believe that pockets of solvent trapped between the two bilayer leaflets can disrupt normal protein function. To overcome this limitation, Montal and Mueller developed a modified deposition technique that eliminates the use of a heavy non-volatile solvent. In this method, the aperture starts out above the water surface, completely separating the two fluid chambers. On the surface of each chamber, a monolayer is formed by applying lipids in a volatile solvent such as chloroform
Chloroform
Chloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous...
and waiting for the solvent to evaporate. The aperture is then lowered through the air-water interface and the two monolayers from the separate chambers are folded down against each other, forming a bilayer across the aperture. The stability issue has proven more difficult to solve. Typically, a black lipid membrane will survive for less than an hour, precluding long-term experiment
Long-term experiment
A long-term experiment is an experimental procedure that runs through a long period of time, in order to test a hypothesis or observe a phenomenon that takes place at an extremely slow rate....
s. This lifetime can be extended by precisely structuring the supporting aperture,chemically crosslinking the lipids or gelling the surrounding solution to mechanically support the bilayer. Work is ongoing in this area and lifetimes of several hours will become feasible.
Supported lipid bilayers (SLB)

One of the clearest examples of this advantage is the use of mechanical probing techniques which require a direct physical interaction with the sample. Atomic force microscopy (AFM) has been used to image lipid phase separation
Lipid bilayer phase behavior
One of the most important properties of a lipid bilayer is the relative mobility of the individual lipid molecules and how this mobility changes with temperature. This response is known as the phase behavior of the bilayer. Broadly, at a given temperature a lipid bilayer can exist in either a...
, formation of transmembrane nanopores followed by single protein molecule adsorption, and protein assembly with sub-nm accuracy without the need for a labeling dye. More recently, AFM has also been used to directly probe the mechanical properties
Lipid bilayer mechanics
Lipid bilayer mechanics is the study of the physical material properties of lipid bilayers, classifying bilayer behavior with stress and strain rather than biochemical interactions. These properties are typically characterized in terms of three mechanical elastic modulus: the area compression...
of single bilayers and to perform force spectroscopy on individual membrane proteins. These studies would be difficult or impossible without the use of supported bilayers since the surface of a cell or vesicle is relatively soft and would drift and fluctuate over time. Another example of a physical probe is the use of the quartz crystal microbalance
Quartz crystal microbalance
A quartz crystal microbalance measures a mass per unit area by measuring the change in frequency of a quartz crystal resonator. The resonance is disturbed by the addition or removal of a small mass due to oxide growth/decay or film deposition at the surface of the acoustic resonator...
(QCM) to study binding kinetics at the bilayer surface. Dual polarisation interferometry
Dual Polarisation Interferometry
Dual polarization interferometry is an analytical technique that can probe molecular scale layers adsorbed to the surface of a waveguide by using the evanescent wave of a laser beam confined to the waveguide...
is a high resolution optical tool for characterising the order and disruption in lipid bilayers during interactions or phase transitions providing complementary data to QCM measurements .
Many modern fluorescence microscopy techniques also require a rigidly-supported planar surface. Evanescent field
Evanescent wave
An evanescent wave is a nearfield standing wave with an intensity that exhibits exponential decay with distance from the boundary at which the wave was formed. Evanescent waves are a general property of wave-equations, and can in principle occur in any context to which a wave-equation applies...
methods such as total internal reflection fluorescence microscopy (TIRF) and surface plasmon resonance
Surface plasmon resonance
The excitation of surface plasmons by light is denoted as a surface plasmon resonance for planar surfaces or localized surface plasmon resonance for nanometer-sized metallic structures....
(SPR) can offer extremely sensitive measurement of analyte binding and bilayer optical properties but can only function when the sample is supported on specialized optically functional materials. Another class of methods applicable only to supported bilayers is those based on optical interference such as fluorescence interference contrast microscopy (FLIC) and reflection interference contrast microscopy (RICM). When the bilayer is supported on top of a reflective surface, variations in intensity due to destructive interference from this interface can be used to calculate with angstrom accuracy the position of fluorophores within the bilayer. Both evanescent and interference techniques offer sub-wavelength resolution in only one dimension (z, or vertical). In many cases, this resolution is all that is needed. After all, bilayers are very small only in one dimension. Laterally, a bilayer can extend for many micrometres or even millimeters. But certain phenomena like dynamic phase rearrangement do occur in bilayers on a lateral sub-micrometre lengthscale. A promising approach to studying these structures is near field scanning optical microscopy (NSOM). Like AFM, NSOM relies on the scanning of a micromachined tip to give a highly localized signal. But unlike AFM, NSOM uses an optical rather than physical interaction with the sample, potentially perturbing delicate structures to a lesser extent.

Microfluidics
Microfluidics deals with the behavior, precise control and manipulation of fluids that are geometrically constrained to a small, typically sub-millimeter, scale.Typically, micro means one of the following features:* small volumes...
to demonstrate that stable composition gradients could be formed in bilayers, potentially allowing massively parallel studies of phase segregation, molecular binding and cellular response to artificial lipid membranes. Creative utilization of the corral concept has also allowed studies of the dynamic reorganization of membrane proteins at the synaptic
Synapse
In the nervous system, a synapse is a structure that permits a neuron to pass an electrical or chemical signal to another cell...
interface.
One of the primary limitations of supported bilayers is the possibility of unwanted interactions with the substrate. Although supported bilayers generally do not directly touch the substrate surface, they are separated by only a very thin water gap. The size and nature of this gap depends on the substrate material and lipid species but is generally about 1 nm for zwitterionic lipids supported on silica, the most common experimental system. Because this layer is so thin there is extensive hydrodynamic coupling between the bilayer and the substrate, resulting in a lower diffusion coefficient in supported bilayers than for free bilayers of the same composition. A certain percentage of the supported bilayer will also be completely immobile, although the exact nature of and reason for these “pinned” sites is still uncertain. For high quality liquid phase supported bilayers the immobile fraction is typically around 1-5%. To quantify the diffusion coefficient and mobile fraction, researchers studying supported bilayers will often report FRAP
Fluorescence recovery after photobleaching
Fluorescence recovery after photobleaching denotes an optical technique capable of quantifying the two dimensional lateral diffusion of a molecularly thin film containing fluorescently labeled probes, or to examine single cells. This technique is very useful in biological studies of cell membrane...
data.
Unwanted substrate interactions are a much greater problem when incorporating integral membrane proteins, particularly those with large domains sticking out beyond the core of the bilayer. Because the gap between bilayer and substrate is so thin these proteins will often become denatured
Denaturation (biochemistry)
Denaturation is a process in which proteins or nucleic acids lose their tertiary structure and secondary structure by application of some external stress or compound, such as a strong acid or base, a concentrated inorganic salt, an organic solvent , or heat...
on the substrate surface and therefore lose all functionality. One approach to circumvent this problem is the use of polymer tethered bilayers. In these systems the bilayer is supported on a loose network of hydrated polymers or hydrogel which acts as a spacer and theoretically prevents denaturing substrate interactions. In practice, some percentage of the proteins will still lose mobility and functionality, probably due to interactions with the polymer/lipid anchors. Research in this area is ongoing.
Tethered Bilayer Lipid Membranes (t-BLM)
The use of a tethered bilayer lipid membrane (t-BLM) further increases the stability supported membranes by chemically anchoring the lipids to the solid substrate providing a greatly enhanced stability .Gold can be used as a substrate because of its inert chemistry and thiolipids for covalent binding to the gold. Thiolipids are composed of lipid derivatives, extended at their polar head-groups by hydrophilic spacers which terminate in a thiol or disulphide group that forms a covalent bond with gold, forming self assembled monolayers (SAM).
The limitation of the intra-membrane mobility of supported lipid bilayers can be overcome by introducing half-membrane spanning tether lipids with benzyl disulphide (DPL) and synthetic archaea analogue full membrane spanning lipids with phytanoly chains to stabilize the structure and polyethyleneglycol units as a hydrophilic spacer. Bilayer formation is achieved either by exposure of the lipid coated gold substrate to outer layer lipids either in an ethanol solution or in liposomes
. The advantage of this approach is that the because of the hydrophilic space of around 4 nm, the interaction with the substrate is minimal and the extra space allows the introduction of protein ion channels into the bilayer. Additionally the spacer layer creates an ionic reservoir . that readily enables ac electrical impedance measurement across the bilayer.
Vesicles

Since artificial SUVs can be made in large quantities they are suitable for bulk material studies such as x-ray diffraction to determine lattice spacing and differential scanning calorimetry to determine phase transitions. Dual polarisation interferometry
Dual Polarisation Interferometry
Dual polarization interferometry is an analytical technique that can probe molecular scale layers adsorbed to the surface of a waveguide by using the evanescent wave of a laser beam confined to the waveguide...
can measure unilamelar and multilamelar structures and insertion into and disruption of the vesciles in a label free assay format . Vesicles can also be labeled with fluorescent dyes to allow sensitive FRET-based fusion
Lipid bilayer fusion
Fusion is the process by which two initially distinct lipid bilayers merge their hydrophobic cores, resulting in one interconnected structure. If this fusion proceeds completely through both leaflets of both bilayers, an aqueous bridge is formed and the internal contents of the two structures can mix...
assays. In spite of this fluorescent labeling it is often difficult to perform detailed imaging on SUVs simply because they are so small. To combat this problem researchers have developed the giant unilamellar vesicle (GUV). GUVs are large enough (several tens of micrometres) to study with traditional florescence microscopy. Many of the studies of lipid rafts in artificial lipid systems have been performed with GUVs for this reason. Compared to supported bilayers, GUVs present a more “natural” environment since there is no nearby solid surface to induce defects or denature proteins. However, GUVs are relatively fragile, time consuming to make and can only be produced in limited yield compared to SUVs.
To circumvent these problems a microfluidic assembly line approach to GUVs was reported.
Micelles, bicelles and Nanodiscs
DetergentDetergent
A detergent is a surfactant or a mixture of surfactants with "cleaning properties in dilute solutions." In common usage, "detergent" refers to alkylbenzenesulfonates, a family of compounds that are similar to soap but are less affected by hard water...
micelles are another class of model membranes that are commonly to purify and study membrane proteins, although they lack a lipid bilayer. In aqueous solutions, micelles are assemblies of amphipathic molecules with their hydrophilic heads exposed to solvent and their hydrophobic tails in the center. Micelles can solubilize membrane proteins by partially encapsulating them and shielding their hydrophobic surfaces from solvent.
Bicelles are a related class of model membrane , typically made of two lipids, one of which forms a lipid bilayer while the other forms an amphipathic, micelle-like assembly shielding the bilayer center from surround solvent molecules. Bicelles can be thought of as a segment of bilayer encapsulated and solubilized by a micelle. Bicelles are much smaller than liposomes, and so can be used in experiments such as NMR
NMR
NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
spectroscopy where the larger vesicles are not an option.
Nanodiscs
Nanodisc
A nanodisc is a synthetic model membrane system which assists in the study of membrane proteins. Nanodiscs are useful for membrane biology in the study of membrane proteins because they represent a more native environment than liposomes, detergent micelles or bicelles and allow the study of...
consist of a segment of bilayer encapsulated by an amphipathic protein coat, rather than a lipid or detergent layer. Nanodiscs are more stable than bicelles and micelles at low concentrations, and are very well-defined in size (depending on the type of protein coat, between 10 and 20 nm). Membrane proteins incorporated into and solubilized by Nanodiscs can be studied by a wide variety of biophysical techniques.

