Lipid bilayer phase behavior
Encyclopedia
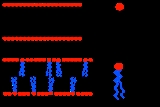
One of the most important properties of a lipid bilayer
is the relative mobility (fluidity) of the individual lipid molecules and how this mobility changes with temperature. This response is known as the phase behavior of the bilayer. Broadly, at a given temperature a lipid bilayer can exist in either a liquid or a solid phase. The solid phase is commonly referred to as a “gel” phase. All lipids have a characteristic temperature at which they undergo a transition (melt
) from the gel to liquid phase. In both phases the lipid molecules are constrained to the two dimensional plane of the membrane, but in liquid phase bilayers the molecules diffuse freely within this plane. Thus, in a liquid bilayer a given lipid will rapidly exchange locations with its neighbor millions of times a second and will, through the process of a random walk
, migrate over long distances.
-based bilayer this process typically occurs over a timescale of weeks. This discrepancy can be understood in terms of the basic structure of the bilayer. For a lipid to flip from one leaflet to the other, its hydrated headgroup must cross the hydrophobic core of the bilayer, an energetically unfavorable process. Unlike liquid phase bilayers, the lipids in a gel phase bilayer are locked in place and exhibit neither flip-flop nor lateral mobility. Due to this limited mobility, gel bilayers lack an important property of liquid bilayers: the ability to reseal small holes. Liquid phase bilayers can spontaneously heal small voids, much the same way a film of oil on water could flow in to fill a gap. This functionality is one of the reasons that cell membranes are usually composed of fluid phase bilayers.
 The phase behavior of lipid bilayers is largely determined by the strength of the attractive Van der Waals
The phase behavior of lipid bilayers is largely determined by the strength of the attractive Van der Waals
interactions between adjacent lipid molecules. The extent of this interaction is in turn governed by how long the lipid
tails are and how well they can pack together. Longer tailed lipids have more area over which to interact, increasing the strength of this interaction and consequently decreasing the lipid mobility. Thus, at a given temperature, a short-tailed lipid will be more fluid than an otherwise identical long-tailed lipid. Another way of expressing this would be to say that the liquid to gel phase transition temperature increases with increasing number of carbons in the lipid alkane
chains. Saturated
phosphatidylcholine lipids with tails longer than 14 carbons are solid at room temperature, while those with fewer than 14 are liquid. This phenomenon is analogous to the fact that paraffin
wax, which is composed of long alkanes, is solid at room temperature, while octane (gasoline
), a short alkane, is liquid.
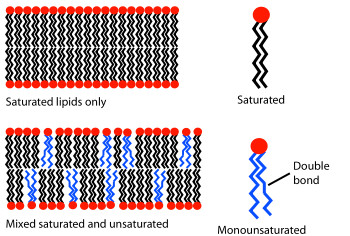
Aside from chain length, transition temperature can also be affected by the degree of unsaturation
of the lipid tails. An unsaturated double bond
can produce a kink in the alkane chain, disrupting the regular periodic structure. This disruption creates extra free space within the bilayer which allows additional flexibility in the adjacent chains. It is this disruption of packing that leads to lower transition temperatures with increasing double bonds. This is a particularly powerful effect; decreasing the overall chain length by one carbon usually alters the transition temperature of a lipid by ten degrees Celsius or less, but adding a single double bond can decrease the transition temperature by seventy degrees or more (see table). An example of this effect can be noted in everyday life as butter
, which has a large percentage saturated fats, is solid at room temperature while vegetable oil, which is mostly unsaturated, is liquid.
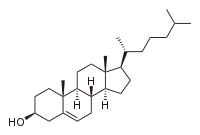 The presence of cholesterol
The presence of cholesterol
exerts a profound but complicated influence on lipid bilayer properties because of its unique physical characteristics. Although it is a lipid, cholesterol bears little resemblance to a phospholipid
. The hydrophilic domain of cholesterol is quite small, consisting of a single alcohol
group. Adjacent to this alcohol is a rigid planar structure composed of several fused rings. At the opposite end of the ring structure is a short single chain tail. It has been known for decades that the addition of cholesterol to a fluid phase bilayer decreases its permeability to water. The mode of this interaction has more recently been shown to be due to cholesterol intercalating between lipid molecules, filling in free space and decreasing the flexibility of surrounding lipid chains. This interaction also increases the mechanical rigidity
of fluid bilayers and decreases their lateral diffusion coefficient. In contrast, the addition of cholesterol to gel phase bilayers disrupts local packing order, increasing the diffusion coefficient and decreasing the elastic modulus. Interactions of cholesterol with multi-component systems are even more complicated, as these can result in intricate phase diagrams. One lipid-cholesterol system that has recently been studied intently is the lipid raft. Lipid rafts are cholesterol-enriched gel domains that have been potentially implicated in certain cell signaling processes, but the subject remains controversial, with some researchers doubting even their existence in vivo.
Lipid bilayer
The lipid bilayer is a thin membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around cells. The cell membrane of almost all living organisms and many viruses are made of a lipid bilayer, as are the membranes surrounding the cell nucleus...
is the relative mobility (fluidity) of the individual lipid molecules and how this mobility changes with temperature. This response is known as the phase behavior of the bilayer. Broadly, at a given temperature a lipid bilayer can exist in either a liquid or a solid phase. The solid phase is commonly referred to as a “gel” phase. All lipids have a characteristic temperature at which they undergo a transition (melt
Melt
Melt can refer to:* Melting, in physics, the process of heating a solid substance to a liquid*Melt , the semi-liquid material used in steelmaking and glassblowing*Melt inclusions, a feature of igneous rock...
) from the gel to liquid phase. In both phases the lipid molecules are constrained to the two dimensional plane of the membrane, but in liquid phase bilayers the molecules diffuse freely within this plane. Thus, in a liquid bilayer a given lipid will rapidly exchange locations with its neighbor millions of times a second and will, through the process of a random walk
Random walk
A random walk, sometimes denoted RW, is a mathematical formalisation of a trajectory that consists of taking successive random steps. For example, the path traced by a molecule as it travels in a liquid or a gas, the search path of a foraging animal, the price of a fluctuating stock and the...
, migrate over long distances.
Motion Constraints
In contrast to this large in-plane mobility, it is very difficult for lipid molecules to flip-flop from one side of the bilayer to the other. In a phosphatidylcholinePhosphatidylcholine
Phosphatidylcholines are a class of phospholipids that incorporate choline as a headgroup.They are a major component of biological membranes and can be easily obtained from a variety of readily available sources such as egg yolk or soy beans from which they are mechanically extracted or chemically...
-based bilayer this process typically occurs over a timescale of weeks. This discrepancy can be understood in terms of the basic structure of the bilayer. For a lipid to flip from one leaflet to the other, its hydrated headgroup must cross the hydrophobic core of the bilayer, an energetically unfavorable process. Unlike liquid phase bilayers, the lipids in a gel phase bilayer are locked in place and exhibit neither flip-flop nor lateral mobility. Due to this limited mobility, gel bilayers lack an important property of liquid bilayers: the ability to reseal small holes. Liquid phase bilayers can spontaneously heal small voids, much the same way a film of oil on water could flow in to fill a gap. This functionality is one of the reasons that cell membranes are usually composed of fluid phase bilayers.
Physical Origins

Van der Waals force
In physical chemistry, the van der Waals force , named after Dutch scientist Johannes Diderik van der Waals, is the sum of the attractive or repulsive forces between molecules other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral...
interactions between adjacent lipid molecules. The extent of this interaction is in turn governed by how long the lipid
Lipid
Lipids constitute a broad group of naturally occurring molecules that include fats, waxes, sterols, fat-soluble vitamins , monoglycerides, diglycerides, triglycerides, phospholipids, and others...
tails are and how well they can pack together. Longer tailed lipids have more area over which to interact, increasing the strength of this interaction and consequently decreasing the lipid mobility. Thus, at a given temperature, a short-tailed lipid will be more fluid than an otherwise identical long-tailed lipid. Another way of expressing this would be to say that the liquid to gel phase transition temperature increases with increasing number of carbons in the lipid alkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
chains. Saturated
Saturation (chemistry)
In chemistry, saturation has six different meanings, all based on reaching a maximum capacity...
phosphatidylcholine lipids with tails longer than 14 carbons are solid at room temperature, while those with fewer than 14 are liquid. This phenomenon is analogous to the fact that paraffin
Paraffin
In chemistry, paraffin is a term that can be used synonymously with "alkane", indicating hydrocarbons with the general formula CnH2n+2. Paraffin wax refers to a mixture of alkanes that falls within the 20 ≤ n ≤ 40 range; they are found in the solid state at room temperature and begin to enter the...
wax, which is composed of long alkanes, is solid at room temperature, while octane (gasoline
Gasoline
Gasoline , or petrol , is a toxic, translucent, petroleum-derived liquid that is primarily used as a fuel in internal combustion engines. It consists mostly of organic compounds obtained by the fractional distillation of petroleum, enhanced with a variety of additives. Some gasolines also contain...
), a short alkane, is liquid.
Aside from chain length, transition temperature can also be affected by the degree of unsaturation
Degree of unsaturation
The degree of unsaturation formula is used in organic chemistry to help draw chemical structures. The formula lets the user determine how many rings, double bonds, and triple bonds are present in the compound to be drawn...
of the lipid tails. An unsaturated double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
can produce a kink in the alkane chain, disrupting the regular periodic structure. This disruption creates extra free space within the bilayer which allows additional flexibility in the adjacent chains. It is this disruption of packing that leads to lower transition temperatures with increasing double bonds. This is a particularly powerful effect; decreasing the overall chain length by one carbon usually alters the transition temperature of a lipid by ten degrees Celsius or less, but adding a single double bond can decrease the transition temperature by seventy degrees or more (see table). An example of this effect can be noted in everyday life as butter
Butter
Butter is a dairy product made by churning fresh or fermented cream or milk. It is generally used as a spread and a condiment, as well as in cooking applications, such as baking, sauce making, and pan frying...
, which has a large percentage saturated fats, is solid at room temperature while vegetable oil, which is mostly unsaturated, is liquid.
| Tail Length | |Transition Temperature | |
|---|---|---|
| 12 | 0 | -1 |
| 14 | 0 | 23 |
| 16 | 0 | 41 |
| 18 | 0 | 55 |
| 20 | 0 | 66 |
| 22 | 0 | 75 |
| 24 | 0 | 80 |
| 18 | 1 | 1 |
| 18 | 2 | -53 |
| 18 | 3 | -60 |
Mixed Systems
Bilayers need not be composed of a single type of lipid and, in fact, most natural membranes are a complex mixture of different lipid molecules. Such mixtures often exhibit properties intermediate to their components, but are also capable of a phenomenon not seen in single component systems: phase separation. If some of the components are liquid at a given temperature while others are in the gel phase, the two phases can coexist in spatially separated populations. This phase separation plays a critical role in biochemical phenomena because membrane components such as proteins can partition into one or the other phase and thus be locally concentrated or activated.Cholesterol

Cholesterol
Cholesterol is a complex isoprenoid. Specifically, it is a waxy steroid of fat that is produced in the liver or intestines. It is used to produce hormones and cell membranes and is transported in the blood plasma of all mammals. It is an essential structural component of mammalian cell membranes...
exerts a profound but complicated influence on lipid bilayer properties because of its unique physical characteristics. Although it is a lipid, cholesterol bears little resemblance to a phospholipid
Phospholipid
Phospholipids are a class of lipids that are a major component of all cell membranes as they can form lipid bilayers. Most phospholipids contain a diglyceride, a phosphate group, and a simple organic molecule such as choline; one exception to this rule is sphingomyelin, which is derived from...
. The hydrophilic domain of cholesterol is quite small, consisting of a single alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
group. Adjacent to this alcohol is a rigid planar structure composed of several fused rings. At the opposite end of the ring structure is a short single chain tail. It has been known for decades that the addition of cholesterol to a fluid phase bilayer decreases its permeability to water. The mode of this interaction has more recently been shown to be due to cholesterol intercalating between lipid molecules, filling in free space and decreasing the flexibility of surrounding lipid chains. This interaction also increases the mechanical rigidity
Lipid bilayer mechanics
Lipid bilayer mechanics is the study of the physical material properties of lipid bilayers, classifying bilayer behavior with stress and strain rather than biochemical interactions. These properties are typically characterized in terms of three mechanical elastic modulus: the area compression...
of fluid bilayers and decreases their lateral diffusion coefficient. In contrast, the addition of cholesterol to gel phase bilayers disrupts local packing order, increasing the diffusion coefficient and decreasing the elastic modulus. Interactions of cholesterol with multi-component systems are even more complicated, as these can result in intricate phase diagrams. One lipid-cholesterol system that has recently been studied intently is the lipid raft. Lipid rafts are cholesterol-enriched gel domains that have been potentially implicated in certain cell signaling processes, but the subject remains controversial, with some researchers doubting even their existence in vivo.

