
Methylisocitrate lyase
Encyclopedia
In enzymology, a methylisocitrate lyase is an enzyme
that catalyzes
the chemical reaction
-3-hydroxybutane-1,2,3-tricarboxylate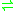 pyruvate + succinate
pyruvate + succinate
Hence, this enzyme has one substrate
, (2S,3R)-3-hydroxybutane-1,2,3-tricarboxylate (also known as 2-methylisocitrate), and two products
, pyruvate and succinate.
The reaction is similar to that of isocitrate lyase
, except that an additional methyl group (marked with an asterisk in the above scheme) is present, meaning that citrate
is replaced by methylcitrate and glyoxylate by pyruvate.
This enzyme belongs to the family of lyase
s, specifically the oxo-acid-lyases, which cleave carbon-carbon bonds. The systematic name of this enzyme class is (2S,3R)-3-hydroxybutane-1,2,3-tricarboxylate pyruvate-lyase (succinate-forming). Other names in common use include 2-methylisocitrate lyase, MICL, and (2S,3R)-3-hydroxybutane-1,2,3-tricarboxylate pyruvate-lyase. This enzyme participates in propanoate metabolism.
Methylisocitrate lyase was discovered in 1976.
have been solved for this class of enzymes, with PDB
accession codes , , , , , and . The structure is very similar to that of phosphoenolpyruvate mutase
. A homotetrameric biological unit is composed of beta barrels with the active site at one end. A magnesium ion is present in the active site, and an active-site "gating loop" moves inward toward it when substrate binds and away with no substrate bound, thus shielding the reaction from solvent. Helices are present all around the beta barrels; in particular, a C-terminal helical domain splits off from the barrel to interact with the barrel of a neighboring subunit, in a "helix swapping" motif (see phosphoenolpyruvate mutase
).
The following still shot from a ribbon
kinemage
shows one subunit from the crystal structure 1MUM, which includes a magnesium ion (gray) but no substrate; helices are red while loops are white and beta strands are green.
instead of acetyl coenzyme A. The enzyme 2-methylcitrate synthase
adds propionyl coenzyme A to oxaloacetate, yielding methylcitrate instead of citrate
. But isomerizing methylcitrate to methylisocitrate and then subjecting it to MICL regenerates succinate, which proceeds as in the Krebs cycle, and pyruvate, which is easily metabolized by other pathways (e.g. decarboxylated to form acetyl coenzyme A and oxidized in the Krebs cycle). This allows catabolism of propionic acid -- and, using beta oxidation
, other fatty acids with odd numbers of carbons -- without relying on coenzyme B12, a complex cofactor often used to metabolize propionate. The methylcitrate cycle is found in many microorganism
s.
Methylisocitrate lyase plays a regulatory function in this cycle; it is activated by NAD
but inhibited noncompetitively
by NADH and NADPH.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
-3-hydroxybutane-1,2,3-tricarboxylate
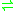 pyruvate + succinate
pyruvate + succinateHence, this enzyme has one substrate
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
, (2S,3R)-3-hydroxybutane-1,2,3-tricarboxylate (also known as 2-methylisocitrate), and two products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
, pyruvate and succinate.
The reaction is similar to that of isocitrate lyase
Isocitrate lyase
Isocitrate lyase , or ICL, is an enzyme in the glyoxylate cycle that catalyzes the cleavage of isocitrate to succinate and glyoxylate. Together with malate synthase, it bypasses the two decarboxylation steps of the tricarboxylic acid cycle and is used by bacteria, fungi, and plants.The systematic...
, except that an additional methyl group (marked with an asterisk in the above scheme) is present, meaning that citrate
Citrate
A citrate can refer either to the conjugate base of citric acid, , or to the esters of citric acid. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate.-Other citric acid ions:...
is replaced by methylcitrate and glyoxylate by pyruvate.
This enzyme belongs to the family of lyase
Lyase
In biochemistry, a lyase is an enzyme that catalyzes the breaking of various chemical bonds by means other than hydrolysis and oxidation, often forming a new double bond or a new ring structure...
s, specifically the oxo-acid-lyases, which cleave carbon-carbon bonds. The systematic name of this enzyme class is (2S,3R)-3-hydroxybutane-1,2,3-tricarboxylate pyruvate-lyase (succinate-forming). Other names in common use include 2-methylisocitrate lyase, MICL, and (2S,3R)-3-hydroxybutane-1,2,3-tricarboxylate pyruvate-lyase. This enzyme participates in propanoate metabolism.
Methylisocitrate lyase was discovered in 1976.
Structural studies
As of late 2007, 6 structuresTertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
have been solved for this class of enzymes, with PDB
Protein Data Bank
The Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids....
accession codes , , , , , and . The structure is very similar to that of phosphoenolpyruvate mutase
Phosphoenolpyruvate mutase
In enzymology, a phosphoenolpyruvate mutase is an enzyme that catalyzes the chemical reactionHence, this enzyme has one substrate, phosphoenolpyruvate , and one product, 3-phosphonopyruvate , which are structural isomers....
. A homotetrameric biological unit is composed of beta barrels with the active site at one end. A magnesium ion is present in the active site, and an active-site "gating loop" moves inward toward it when substrate binds and away with no substrate bound, thus shielding the reaction from solvent. Helices are present all around the beta barrels; in particular, a C-terminal helical domain splits off from the barrel to interact with the barrel of a neighboring subunit, in a "helix swapping" motif (see phosphoenolpyruvate mutase
Phosphoenolpyruvate mutase
In enzymology, a phosphoenolpyruvate mutase is an enzyme that catalyzes the chemical reactionHence, this enzyme has one substrate, phosphoenolpyruvate , and one product, 3-phosphonopyruvate , which are structural isomers....
).
The following still shot from a ribbon
Ribbon diagram
Proteins are biological macromolecules made up of a long polypeptide chain of amino acids linked by peptide bonds...
kinemage
Kinemage
A kinemage is an interactive graphic scientific illustration. It often is used to visualize molecules, especially proteins and nucleic acids, although it can also represent other types of 3-dimensional data...
shows one subunit from the crystal structure 1MUM, which includes a magnesium ion (gray) but no substrate; helices are red while loops are white and beta strands are green.
Biological function
Methylisocitrate lyase is used in the methylcitrate cycle, a modified version of the Krebs cycle that metabolizes propionyl coenzyme ACoenzyme A
Coenzyme A is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All sequenced genomes encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it as a substrate...
instead of acetyl coenzyme A. The enzyme 2-methylcitrate synthase
2-methylcitrate synthase
In enzymology, a 2-methylcitrate synthase is an enzyme that catalyzes the chemical reactionThe 3 substrates of this enzyme are propanoyl-CoA, H2O, and oxaloacetate, whereas its two products are -2-hydroxybutane-1,2,3-tricarboxylate and CoA....
adds propionyl coenzyme A to oxaloacetate, yielding methylcitrate instead of citrate
Citrate
A citrate can refer either to the conjugate base of citric acid, , or to the esters of citric acid. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate.-Other citric acid ions:...
. But isomerizing methylcitrate to methylisocitrate and then subjecting it to MICL regenerates succinate, which proceeds as in the Krebs cycle, and pyruvate, which is easily metabolized by other pathways (e.g. decarboxylated to form acetyl coenzyme A and oxidized in the Krebs cycle). This allows catabolism of propionic acid -- and, using beta oxidation
Beta oxidation
Beta oxidation is the process by which fatty acids, in the form of Acyl-CoA molecules, are broken down in mitochondria and/or in peroxisomes to generate Acetyl-CoA, the entry molecule for the Citric Acid cycle....
, other fatty acids with odd numbers of carbons -- without relying on coenzyme B12, a complex cofactor often used to metabolize propionate. The methylcitrate cycle is found in many microorganism
Microorganism
A microorganism or microbe is a microscopic organism that comprises either a single cell , cell clusters, or no cell at all...
s.
Methylisocitrate lyase plays a regulatory function in this cycle; it is activated by NAD
NAD
NAD may refer to:* No abnormality detected, a medical status description* No apparent distress, a status description in childbirth* NAD Electronics, a Canadian audio equipment manufacturer...
but inhibited noncompetitively
Non-competitive inhibition
Non-competitive inhibition is a type of enzyme inhibition where the inhibitor reduces the activity of the enzyme, by binding not to the active site on the enzyme, but to a different site...
by NADH and NADPH.

