Methemoglobinemia
Encyclopedia
Methemoglobinemia is a disorder characterized by the presence of a higher than normal level of methemoglobin (metHb) in the blood
. Methemoglobin is an oxidized form of hemoglobin
that has an increased affinity for oxygen, resulting in a reduced ability to release oxygen to tissues. The oxygen–hemoglobin dissociation curve is shifted to the left. When methemoglobin concentration is elevated in red blood cells, tissue hypoxia can occur.
within the red blood cell are overwhelmed and the oxygen carrying ferrous ion (Fe2+)
of the heme
group of the hemoglobin molecule is oxidized to the ferric state (Fe3+)
. This converts hemoglobin to methemoglobin, resulting in a reduced ability to release oxygen to tissues and thereby hypoxia. This can give the blood a bluish or chocolate-brown color. Spontaneous formation of methemoglobin is normally reduced (via electron donation) by protective enzyme systems, e.g. NADH methemoglobin reductase (cytochrome-b5 reductase) (major pathway), NADPH methemoglobin reductase (minor pathway) and to a lesser extent the ascorbic acid and glutathione enzyme systems. Disruptions with these enzyme systems lead to the condition.
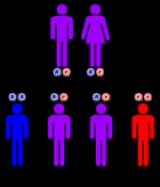
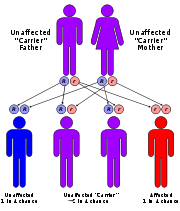 Due to a deficiency of the enzyme
Due to a deficiency of the enzyme
diaphorase I (NADH methemoglobin reductase), methemoglobin levels rise and the blood of met-Hb patients has reduced oxygen-carrying capacity. Instead of being red in color, the arterial blood of met-Hb patients is brown. This results in the skin of Caucasian
patients gaining a bluish hue. Hereditary met-Hb is caused by a recessive gene. If only one parent has this gene, offspring will have normal-hued skin, but if both parents carry the gene there is a chance the offspring will have blue-hued skin.
Another cause of congenital methemoglobinemia is seen in patients with abnormal hemoglobin variants such as hemoglobin M (HbM), or hemoglobin H (HbH), which are not amenable to reduction
despite intact enzyme systems.
Methemoglobinemia can also arise in patients with pyruvate kinase deficiency
due to impaired production of NADH – the essential cofactor for diaphorase I. Similarly, patients with Glucose-6-phosphate dehydrogenase (G6PD) deficiency
may have impaired production of another co-factor, NADPH.
, dapsone
and nitrate
s) may accelerate the rate of formation of methemoglobin up to one-thousandfold, overwhelming the protective enzyme systems and acutely increasing methemoglobin levels.
Other classical drug causes of methemoglobinemia include antibiotic
s (trimethoprim
, sulfonamides
and dapsone
), local anesthetic
s (especially articaine and prilocaine
), and others such as aniline
dyes, metoclopramide
, chlorate
s and bromate
s. Ingestion of compounds containing nitrates (such as the patina chemical bismuth nitrate) can also cause methemoglobinemia.
Infants under 6 months of age are particularly susceptible to methemoglobinemia caused by nitrates ingested in drinking water (called blue-baby syndrome), dehydration usually caused by gastroenteritis with diarrhea, sepsis, and topical anesthetics containing benzocaine or prilocaine. Nitrates used in agricultural fertilizers may leak into the ground and may contaminate well water. The current EPA standard of 10 ppm nitrate-nitrogen for drinking water is specifically designed to protect infants.
Benzocaine
applied to the gums or throat (as commonly used in baby teething
gels) can cause methemoglobinemia.http://www.fda.gov/Drugs/DrugSafety/ucm250024.htm
1% solution (10 mg/ml) 1 to 2 mg/kg administered intravenously slowly over five minutes followed by IV flush with normal saline. Methylene blue restores the iron in hemoglobin to its normal (reduced
) oxygen-carrying state.
This is achieved by providing an artificial electron acceptor (such as methylene blue, or flavin) for NADPH methemoglobin reductase (RBCs usually don't have one; the presence of methylene blue allows the enzyme to function at 5x normal levels) The NADPH is generated via the hexose monophosphate shunt.
Diaphorase II normally contributes only a small percentage of the red blood cells reducing capacity but is pharmacologically activated by exogenous cofactors, such as methylene blue, to 5 times its normal level of activity. Genetically induced chronic low-level methemoglobinemia may be treated with oral methylene blue daily. Also, vitamin C can occasionally reduce cyanosis associated with chronic methemoglobinemia but has no role in treatment of acute acquired methemoglobinemia.
, mental status changes (~50%), headache, fatigue, exercise intolerance, dizziness and loss of consciousness. Arterial blood with elevated methemoglobin levels has a characteristic chocolate-brown color as compared to normal bright red oxygen containing arterial blood.
Severe methemoglobinemia (methemoglobin >50%) patients have dysrhythmias, seizures, coma and death (>70%). Healthy people may not have many symptoms with methemoglobin levels < 15%, however patients with co-morbidities such as anemia, cardiovascular disease, lung disease, sepsis, or presence of other abnormal hemoglobin species (e.g. carboxyhemoglobin, sulfehemoglobin or sickle hemoglobin) may experience moderate to severe symptoms at much lower levels (as low as 5-8%).
, circa 1800. His wife was a carrier of the recessive methemoglobinemia (met-H) gene
, as was a nearby clan with whom the Fugates intermarried. As a result, many descendants of the Fugates were born with met-H.
The "blue men of Lurgan
" were a pair of Lurgan men suffering from what was described as "familial idiopathic
methaemoglobinaemia" who were treated by Dr. James Deeny in 1942. Deeny, who would later become the Chief Medical Officer of the Republic of Ireland
, prescribed a course of ascorbic acid
and sodium bicarbonate
. In case one, by the eighth day of treatment there was a marked change in appearance and by the twelfth day of treatment the patient's complexion was normal. In case two, the patient's complexion reached normality over a month-long duration of treatment.
Blood
Blood is a specialized bodily fluid in animals that delivers necessary substances such as nutrients and oxygen to the cells and transports metabolic waste products away from those same cells....
. Methemoglobin is an oxidized form of hemoglobin
Hemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
that has an increased affinity for oxygen, resulting in a reduced ability to release oxygen to tissues. The oxygen–hemoglobin dissociation curve is shifted to the left. When methemoglobin concentration is elevated in red blood cells, tissue hypoxia can occur.
Overview
Normally, methemoglobin levels are <1%, as measured by the co-oximetry test. Elevated levels of methemoglobin in the blood are caused when the mechanisms that defend against oxidative stressOxidative stress
Oxidative stress represents an imbalance between the production and manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage...
within the red blood cell are overwhelmed and the oxygen carrying ferrous ion (Fe2+)
Ferrous
Ferrous , in chemistry, indicates a divalent iron compound , as opposed to ferric, which indicates a trivalent iron compound ....
of the heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
group of the hemoglobin molecule is oxidized to the ferric state (Fe3+)
Ferric
Ferric refers to iron-containing materials or compounds. In chemistry the term is reserved for iron with an oxidation number of +3, also denoted iron or Fe3+. On the other hand, ferrous refers to iron with oxidation number of +2, denoted iron or Fe2+...
. This converts hemoglobin to methemoglobin, resulting in a reduced ability to release oxygen to tissues and thereby hypoxia. This can give the blood a bluish or chocolate-brown color. Spontaneous formation of methemoglobin is normally reduced (via electron donation) by protective enzyme systems, e.g. NADH methemoglobin reductase (cytochrome-b5 reductase) (major pathway), NADPH methemoglobin reductase (minor pathway) and to a lesser extent the ascorbic acid and glutathione enzyme systems. Disruptions with these enzyme systems lead to the condition.
Congenital methemoglobinemia

Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
diaphorase I (NADH methemoglobin reductase), methemoglobin levels rise and the blood of met-Hb patients has reduced oxygen-carrying capacity. Instead of being red in color, the arterial blood of met-Hb patients is brown. This results in the skin of Caucasian
White people
White people is a term which usually refers to human beings characterized, at least in part, by the light pigmentation of their skin...
patients gaining a bluish hue. Hereditary met-Hb is caused by a recessive gene. If only one parent has this gene, offspring will have normal-hued skin, but if both parents carry the gene there is a chance the offspring will have blue-hued skin.
Another cause of congenital methemoglobinemia is seen in patients with abnormal hemoglobin variants such as hemoglobin M (HbM), or hemoglobin H (HbH), which are not amenable to reduction
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
despite intact enzyme systems.
Methemoglobinemia can also arise in patients with pyruvate kinase deficiency
Pyruvate kinase deficiency
Pyruvate kinase deficiency, also called erythrocyte pyruvate kinase deficiency, is an inherited metabolic disorder of the enzyme pyruvate kinase which affects the survival of red blood cells and causes them to deform into echinocytes on peripheral blood smears.Both autosomal dominant and recessive...
due to impaired production of NADH – the essential cofactor for diaphorase I. Similarly, patients with Glucose-6-phosphate dehydrogenase (G6PD) deficiency
Glucose-6-phosphate dehydrogenase deficiency
Glucose-6-phosphate dehydrogenase deficiency is an X-linked recessive hereditary disease characterised by abnormally low levels of glucose-6-phosphate dehydrogenase , a metabolic enzyme involved in the pentose phosphate pathway, especially important in red blood cell metabolism. G6PD deficiency is...
may have impaired production of another co-factor, NADPH.
Acquired methemoglobinemia
Methemoglobinemia can also be acquired. The protective enzyme systems normally present in red blood cells maintain methemoglobin levels at less than one percent of the total hemoglobin in healthy people. Exposure to exogenous oxidizing drugs and their metabolites (such as benzocaineBenzocaine
Benzocaine is a local anesthetic commonly used as a topical pain reliever, or in cough drops. It is the active ingredient in many over-the-counter anesthetic ointments...
, dapsone
Dapsone
Dapsone is a medication most commonly used in combination with rifampicin and clofazimine as multidrug therapy for the treatment of Mycobacterium leprae infections . It is also second-line treatment for prophylaxis against Pneumocystis pneumonia caused by Pneumocystis jiroveci Dapsone...
and nitrate
Nitrate
The nitrate ion is a polyatomic ion with the molecular formula NO and a molecular mass of 62.0049 g/mol. It is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically-bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a...
s) may accelerate the rate of formation of methemoglobin up to one-thousandfold, overwhelming the protective enzyme systems and acutely increasing methemoglobin levels.
Other classical drug causes of methemoglobinemia include antibiotic
Antibiotic
An antibacterial is a compound or substance that kills or slows down the growth of bacteria.The term is often used synonymously with the term antibiotic; today, however, with increased knowledge of the causative agents of various infectious diseases, antibiotic has come to denote a broader range of...
s (trimethoprim
Trimethoprim
Trimethoprim is a bacteriostatic antibiotic mainly used in the prophylaxis and treatment of urinary tract infections.It belongs to the class of chemotherapeutic agents known as dihydrofolate reductase inhibitors...
, sulfonamides
Sulfonamide (medicine)
Sulfonamide or sulphonamide is the basis of several groups of drugs. The original antibacterial sulfonamides are synthetic antimicrobial agents that contain the sulfonamide group. Some sulfonamides are also devoid of antibacterial activity, e.g., the anticonvulsant sultiame...
and dapsone
Dapsone
Dapsone is a medication most commonly used in combination with rifampicin and clofazimine as multidrug therapy for the treatment of Mycobacterium leprae infections . It is also second-line treatment for prophylaxis against Pneumocystis pneumonia caused by Pneumocystis jiroveci Dapsone...
), local anesthetic
Local anesthetic
A local anesthetic is a drug that causes reversible local anesthesia, generally for the aim of having local analgesic effect, that is, inducing absence of pain sensation, although other local senses are often affected as well...
s (especially articaine and prilocaine
Prilocaine
Prilocaine is a local anesthetic of the amino amide type first prepared by Claes Tegner and Nils Lofgren. In its injectable form , it is often used in dentistry. It is also often combined with lidocaine as a preparation for dermal anesthesia , for treatment of conditions like paresthesia...
), and others such as aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
dyes, metoclopramide
Metoclopramide
Metoclopramide is an antiemetic and gastroprokinetic agent. It is commonly used to treat nausea and vomiting, to facilitate gastric emptying in people with gastroparesis, and as a treatment for the gastric stasis often associated with migraine headaches.-Medical uses:Metoclopramide is commonly...
, chlorate
Chlorate
The chlorate anion has the formula ClO. In this case, the chlorine atom is in the +5 oxidation state. "Chlorate" can also refer to chemical compounds containing this anion; chlorates are the salts of chloric acid. "Chlorate", when followed by a roman numeral in parentheses, e.g...
s and bromate
Bromate
The bromate anion, BrO, is a bromine-based oxoanion. A bromate is a chemical compound that contains this ion. Examples of bromates include sodium bromate, , and potassium bromate, .Bromates are formed many different ways in municipal drinking water...
s. Ingestion of compounds containing nitrates (such as the patina chemical bismuth nitrate) can also cause methemoglobinemia.
Infants under 6 months of age are particularly susceptible to methemoglobinemia caused by nitrates ingested in drinking water (called blue-baby syndrome), dehydration usually caused by gastroenteritis with diarrhea, sepsis, and topical anesthetics containing benzocaine or prilocaine. Nitrates used in agricultural fertilizers may leak into the ground and may contaminate well water. The current EPA standard of 10 ppm nitrate-nitrogen for drinking water is specifically designed to protect infants.
Benzocaine
Benzocaine
Benzocaine is a local anesthetic commonly used as a topical pain reliever, or in cough drops. It is the active ingredient in many over-the-counter anesthetic ointments...
applied to the gums or throat (as commonly used in baby teething
Teething
Teething is the process by which an infant's first teeth sequentially appear by emerging through the gums. Teething may start as early as three months or as late, in some cases, as twelve months. The typical time frame for the first teeth to appear is somewhere between six and nine months...
gels) can cause methemoglobinemia.http://www.fda.gov/Drugs/DrugSafety/ucm250024.htm
Treatment
Methemoglobinemia can be treated with supplemental oxygen and methylene blueMethylene blue
Methylene blue is a heterocyclic aromatic chemical compound with the molecular formula C16H18N3SCl. It has many uses in a range of different fields, such as biology and chemistry. At room temperature it appears as a solid, odorless, dark green powder, that yields a blue solution when dissolved in...
1% solution (10 mg/ml) 1 to 2 mg/kg administered intravenously slowly over five minutes followed by IV flush with normal saline. Methylene blue restores the iron in hemoglobin to its normal (reduced
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
) oxygen-carrying state.
This is achieved by providing an artificial electron acceptor (such as methylene blue, or flavin) for NADPH methemoglobin reductase (RBCs usually don't have one; the presence of methylene blue allows the enzyme to function at 5x normal levels) The NADPH is generated via the hexose monophosphate shunt.
Diaphorase II normally contributes only a small percentage of the red blood cells reducing capacity but is pharmacologically activated by exogenous cofactors, such as methylene blue, to 5 times its normal level of activity. Genetically induced chronic low-level methemoglobinemia may be treated with oral methylene blue daily. Also, vitamin C can occasionally reduce cyanosis associated with chronic methemoglobinemia but has no role in treatment of acute acquired methemoglobinemia.
Symptoms
Signs and symptoms of methemoglobinemia (methemoglobin >1%) include shortness of breath, cyanosisCyanosis
Cyanosis is the appearance of a blue or purple coloration of the skin or mucous membranes due to the tissues near the skin surface being low on oxygen. The onset of cyanosis is 2.5 g/dL of deoxyhemoglobin. The bluish color is more readily apparent in those with high hemoglobin counts than it is...
, mental status changes (~50%), headache, fatigue, exercise intolerance, dizziness and loss of consciousness. Arterial blood with elevated methemoglobin levels has a characteristic chocolate-brown color as compared to normal bright red oxygen containing arterial blood.
Severe methemoglobinemia (methemoglobin >50%) patients have dysrhythmias, seizures, coma and death (>70%). Healthy people may not have many symptoms with methemoglobin levels < 15%, however patients with co-morbidities such as anemia, cardiovascular disease, lung disease, sepsis, or presence of other abnormal hemoglobin species (e.g. carboxyhemoglobin, sulfehemoglobin or sickle hemoglobin) may experience moderate to severe symptoms at much lower levels (as low as 5-8%).
Carriers
The Fugates, a family that lived in the hills of Kentucky, are the most famous example of this hereditary genetic condition. They are known as the "Blue Fugates." Martin Fugate settled near Hazard, KentuckyHazard, Kentucky
As of the census of 2000, there were 4,806 people, 1,946 households, and 1,266 families residing in the city. The population density was 684.6 people per square mile . There were 2,291 housing units at an average density of 326.4 per square mile...
, circa 1800. His wife was a carrier of the recessive methemoglobinemia (met-H) gene
Gene
A gene is a molecular unit of heredity of a living organism. It is a name given to some stretches of DNA and RNA that code for a type of protein or for an RNA chain that has a function in the organism. Living beings depend on genes, as they specify all proteins and functional RNA chains...
, as was a nearby clan with whom the Fugates intermarried. As a result, many descendants of the Fugates were born with met-H.
The "blue men of Lurgan
Lurgan
Lurgan is a town in County Armagh, Northern Ireland. The town is near the southern shore of Lough Neagh and in the north-eastern corner of the county. Part of the Craigavon Borough Council area, Lurgan is about 18 miles south-west of Belfast and is linked to the city by both the M1 motorway...
" were a pair of Lurgan men suffering from what was described as "familial idiopathic
Idiopathic
Idiopathic is an adjective used primarily in medicine meaning arising spontaneously or from an obscure or unknown cause. From Greek ἴδιος, idios + πάθος, pathos , it means approximately "a disease of its own kind". It is technically a term from nosology, the classification of disease...
methaemoglobinaemia" who were treated by Dr. James Deeny in 1942. Deeny, who would later become the Chief Medical Officer of the Republic of Ireland
Republic of Ireland
Ireland , described as the Republic of Ireland , is a sovereign state in Europe occupying approximately five-sixths of the island of the same name. Its capital is Dublin. Ireland, which had a population of 4.58 million in 2011, is a constitutional republic governed as a parliamentary democracy,...
, prescribed a course of ascorbic acid
Ascorbic acid
Ascorbic acid is a naturally occurring organic compound with antioxidant properties. It is a white solid, but impure samples can appear yellowish. It dissolves well in water to give mildly acidic solutions. Ascorbic acid is one form of vitamin C. The name is derived from a- and scorbutus , the...
and sodium bicarbonate
Sodium bicarbonate
Sodium bicarbonate or sodium hydrogen carbonate is the chemical compound with the formula Na HCO3. Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda . The natural mineral form is...
. In case one, by the eighth day of treatment there was a marked change in appearance and by the twelfth day of treatment the patient's complexion was normal. In case two, the patient's complexion reached normality over a month-long duration of treatment.
External links
- ATSDR Case Studies in Environmental Medicine: Nitrate/Nitrite Toxicity U.S. Department of Health and Human Services (public domain)
- The Blue People of Troublesome Creek

